Oral dosage form
A preparation, tablet technology, applied in the production of the composition, mucoadhesive dosage form, attenuated probiotics, prepared mucoadhesive ingredients are fixed to the oral cavity, a system for testing the mucoadhesive properties of the dosage form, oral cavity Dispersible tablets, which maintain a sufficient amount of probiotics, can deliver active substances in the oral cavity, and can solve the problems of no company reporting relevant information and research progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
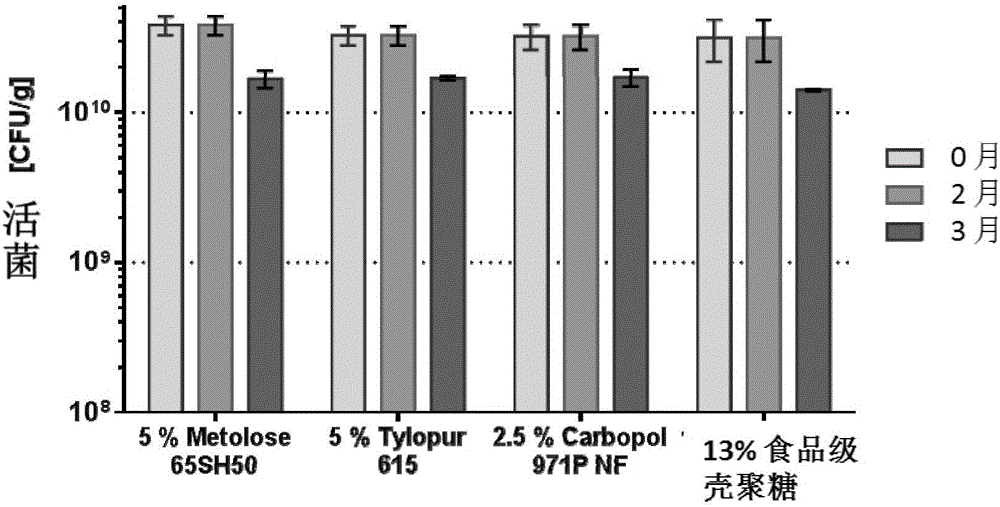
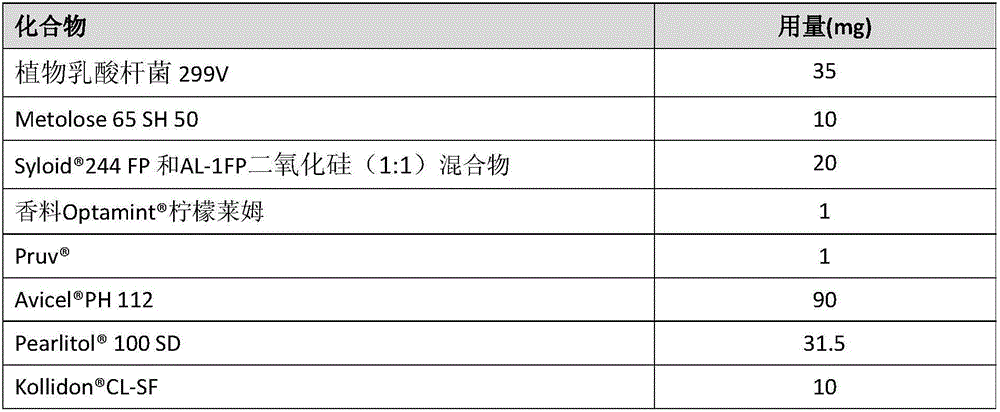
[0127] Preparation of mucoadhesive orodispersible tablets containing granules of a mucoadhesive (Metolose 65SH50) probiotic (Lactobacillus plantarum 299V)
[0128] Preparation of mucoadhesive probiotic particles. First screen Lactobacillus plantarum 299V Particles >200 μm in diameter are removed. will then be sieved and Metolose 65SH50 were added to the Turbula mixer and mixed at 30 rpm for 5 minutes after adding the desiccant bag to obtain a dry homogeneous mixture, which was then transferred to a Somakon experimental mixer and adjusted to 20 ° C under the passage of dry nitrogen. Add 5 ml of Eudragit L isopropanol solution (12.5%) to the mixture with stirring at 280 rpm, then stir the wetted mixture with a scraper for 1 minute, and finally dissolve the remaining Eudragit L isopropanol solution with stirring at 330 rpm. join in. Wet granules were formed after stirring with a scraper at 370 rpm for 2 minutes and sieved through a 710 μm sieve, then placed in a desiccator ...
Embodiment 2
[0135] Preparation of mucoadhesive orodispersible tablets containing granules of a mucoadhesive (Metolose 65SH50) probiotic (Lactobacillus paracasei 8700:2)
[0136] The preparation steps were as in Example 1, but the bacterial powder was not sieved before granulation. The amounts of various materials used are listed in Table 2.
[0137] Table 2: Tablet Composition
[0138]
[0139] The mucoadhesive orodispersible tablet Ex2 was obtained in Example 2 and the tablet characterization data are listed in Table 4.
Embodiment 3
[0143] Preparation of Mucoadhesive Orodispersible Tablets Containing Granules of Mucoadhesive (Food Grade Chitosan) Probiotic (Lactobacillus paracasei 8700:2)
[0144] The preparation steps were as in Example 1, but the bacterial powder was not sieved before granulation. The amounts of various materials used are listed in Table 3.
[0145] Table 3: Tablet Composition
[0146]
[0147]
[0148] In Example 3, the mucoadhesive orodispersible tablet Ex3 was obtained and the tablet characterization data are listed in Table 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com