Zolpidem-based orodispersible pharmaceutical tablet
a technology of orodispersible pharmaceutical tablets and zolpidem, which is applied in the directions of drug compositions, aerosol delivery, inorganic non-active ingredients, etc., can solve the problems of large variability between individuals, development of any new formulation comprising zolpidem is particularly problematic, and the development of a formulation which makes it possible both rapid disintegration and easy detection of misus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0103]Orodispersible Zolpidem Tartrate Tablets, Dosage 10 mg
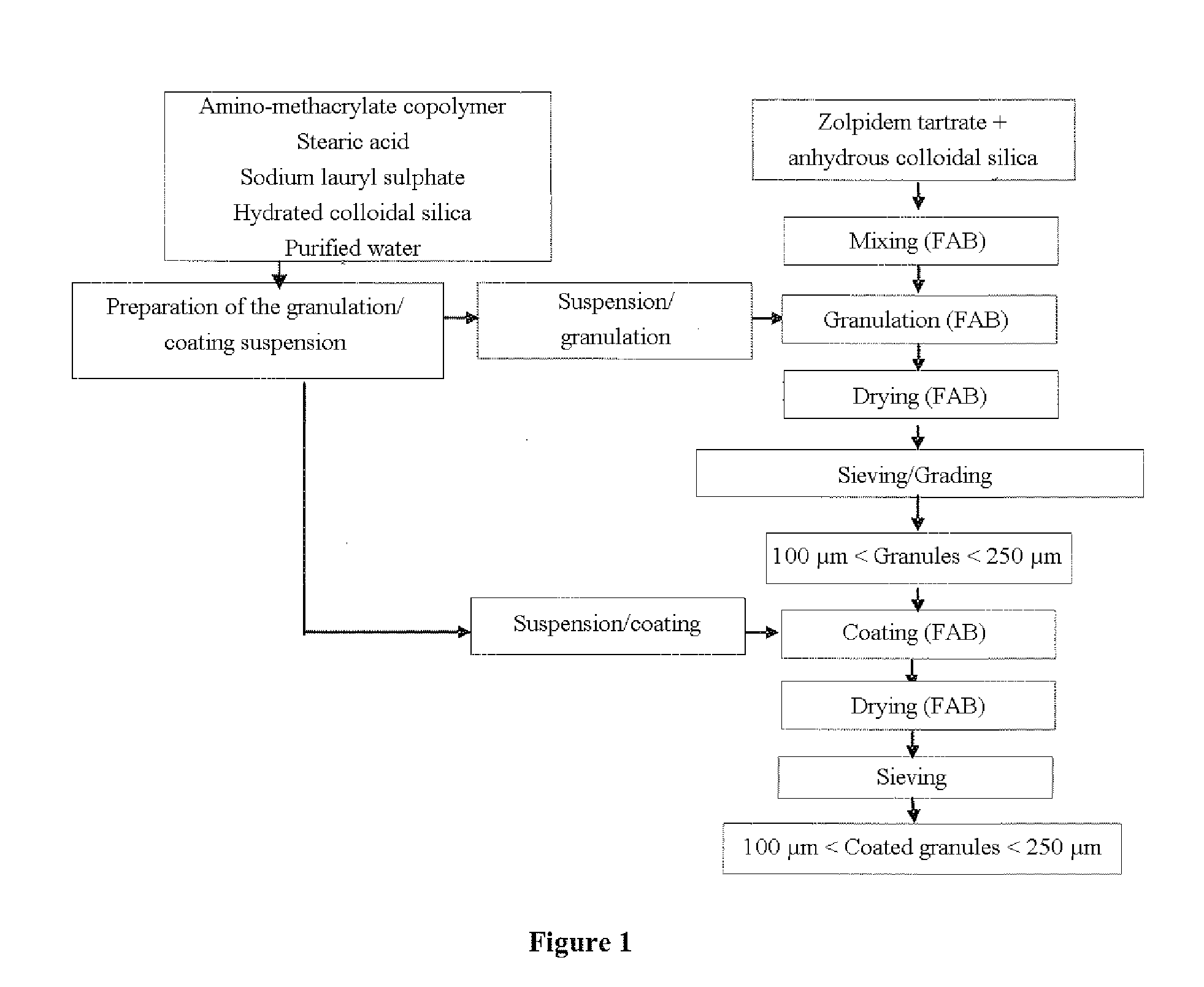
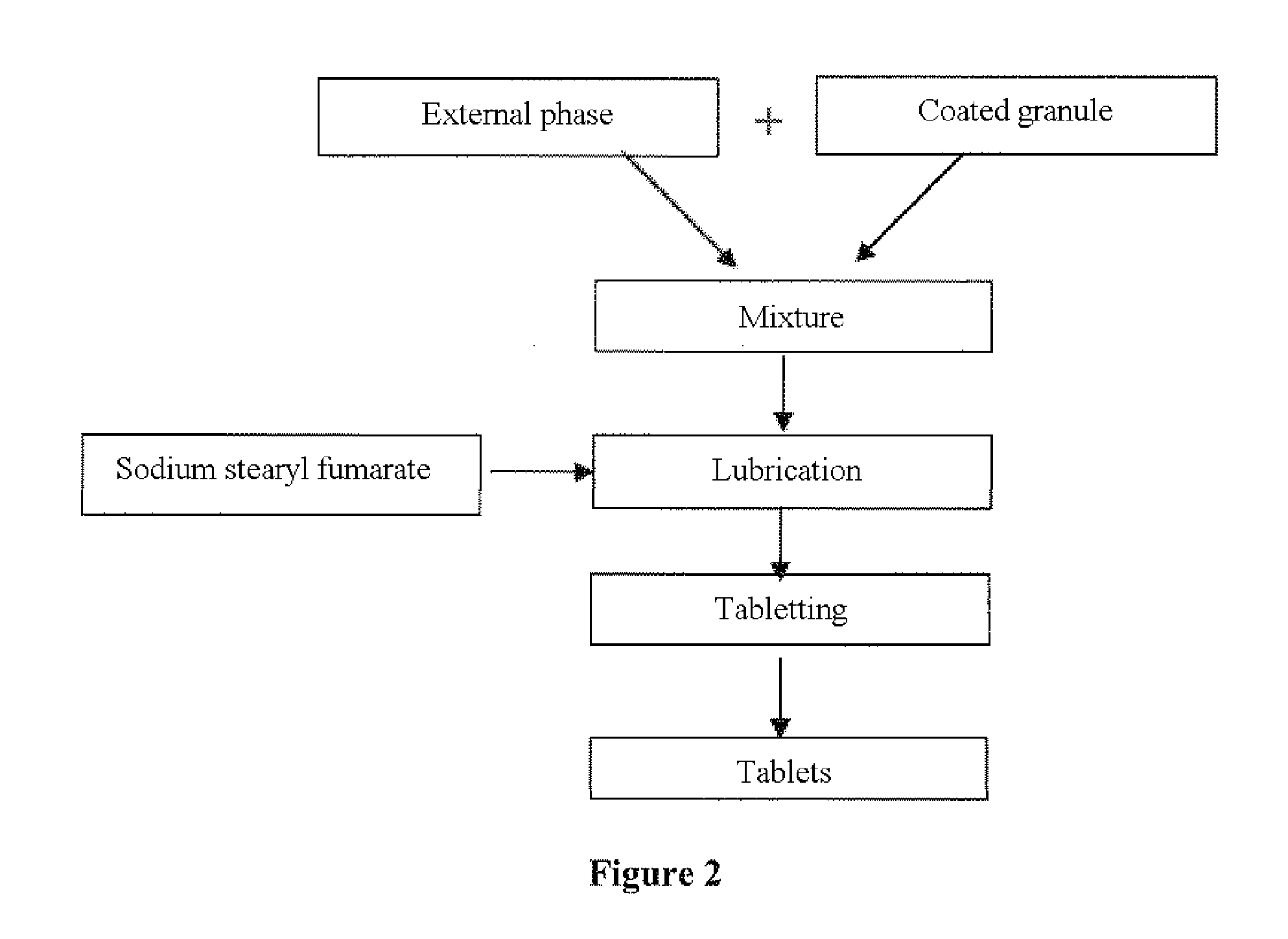
[0104]Manufacturing Process (see FIGS. 1 and 2):
[0105]Zolpidem tartrate is mixed with anhydrous colloidal silica in a fluidized bed. The mixture is subsequently granulated in a fluidized bed using a suspension of amino-methacrylate copolymer. This suspension is preprepared by dispersing amino-methacrylate copolymer, sodium lauryl sulfate, stearic acid and hydrated colloidal silica in purified water, according to the recommendations of the supplier of amino-methacrylate copolymer.
TABLE 1UnitPercentageformulaformulaComposition(mg)(%)Zolpidem tartrate10.004.00Anhydrous colloidal silica (Aerosil ® 200, sold by0.200.08Evonik Degussa GmbH ®)Amino-methacrylate copolymer (Eudragit ® EPO,13.905.56sold by Evonik Degussa GmbH ®)Sodium lauryl sulfate (sold by Cognis ®)1.380.55Stearic acid (Stearic Acid 2236 ®, sold by2.060.83Mallinckrodt Baker ®)Hydrated colloidal silica (Syloïd ® FP 244, sold by4.821.93GRACE Davison ®)Mannitol (Pearli...
example 2
[0115]Orodispersible Zolpidem Tartrate Tablets, Dosage 5 mg
[0116]The orodispersible zolpidem tartrate tablets, dosed at 5 mg, are manufactured according to the same manufacturing process as that described in Example 1.
[0117]The composition is described in detail in the following Table 3.
TABLE 3Unit formulaPercentageComposition(mg)formula (%)Zolpidem tartrate5.002.50Anhydrous colloidal silica (Aerosil ® 200,0.100.05sold by Evonik Degussa GmbH ®)Amino-methacrylate copolymer (Eudragit ®6.933.47EPO, sold by Evonik Degussa GmbH ®)Sodium lauryl sulfate (sold by Cognis ®)0.680.34Stearic acid (Stearic Acid 2236 ®, sold by1.030.51Mallinckrodt Baker ®)Hydrated colloidal silica (Syloïd ® FP 244,2.401.20sold by GRACE Davison ®)Mannitol (Pearlitol ® 160C, sold by71.8635.92Roquette ®, and / or Parteck M200, sold byMerck ®)Crosslinked polyvinylpyrrolidone20.0010.00(Kollidon ® CL, sold by ISP ®)Aspartame (sold by Ajinomoto sweeteners3.001.50Europe SAS)Sodium stearyl fumarate (Pruv ®, sold by3.001.50J...
example 3
[0119]Orodispersible Zolpidem Tartrate Tablets, Dosage 2.5 mg
[0120]The orodispersible zolpidem tartrate tablets, dosed at 2.5 mg, are manufactured according to the same manufacturing process as that described in Example 1.
[0121]The composition is described in detail in the following Table 4.
TABLE 4Unit formulaPercentageComposition(mg)formula (%)Zolpidem tartrate2.501.67Anhydrous colloidal silica (Aerosil ® 200,0.050.03sold by Evonik Degussa GmbH ®)Amino-methacrylate copolymer(Eudragit ®3.472.32EPO, sold by Evonik Degussa GmbH ®)Sodium lauryl sulphate (sold by Cognis ®)0.340.23Stearic acid (Stearic Acid 2236 ®, sold by0.520.34Mallinckrodt Baker ®)Hydrated colloidal silica (Syloïd ® FP 244,1.200.80sold by GRACE Davison ®)Mannitol (Pearlitol ® 160C, sold by50.4033.6Roquette ®, and / or Parteck M200, sold byMerck ®)Crosslinked polyvinylpyrrolidone15.0010.00(Kollidon ® CL, sold by ISP ®)Aspartame (sold by Ajinomoto sweeteners2.251.50Europe SAS)Sodium stearyl fumarate (Pruv ®, sold by2.251....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| mean particle diameter | aaaaa | aaaaa |
| mean particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com