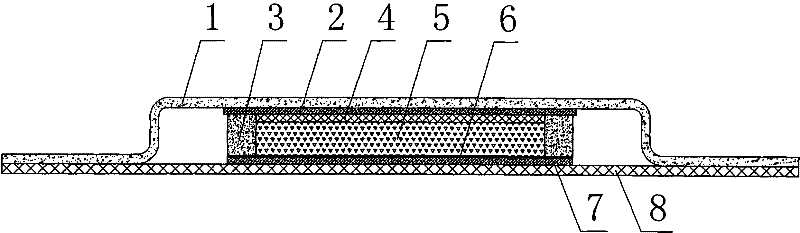
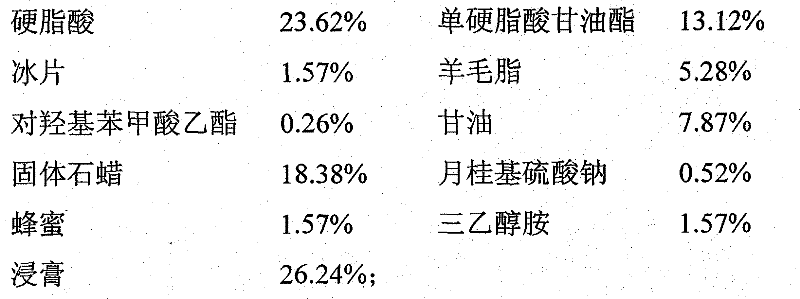
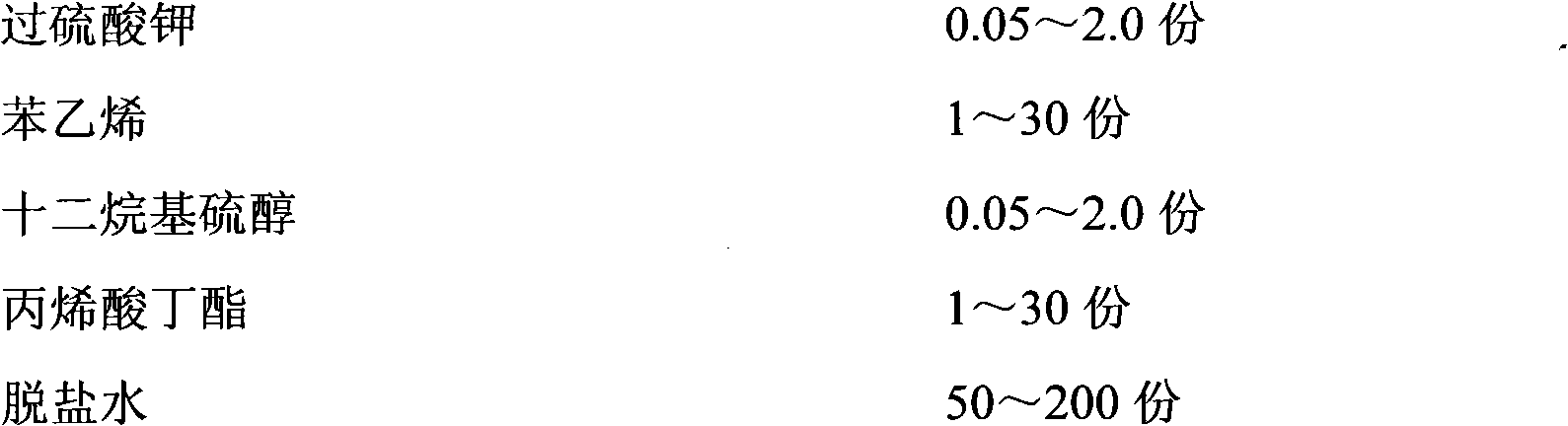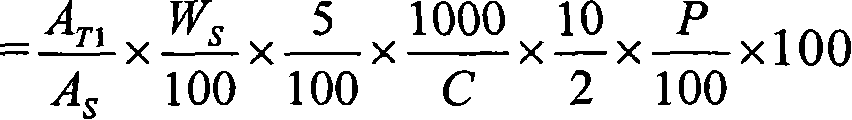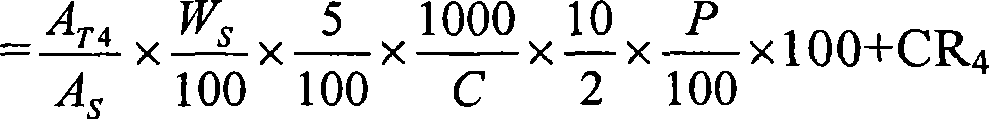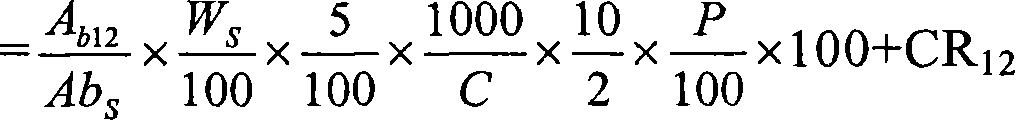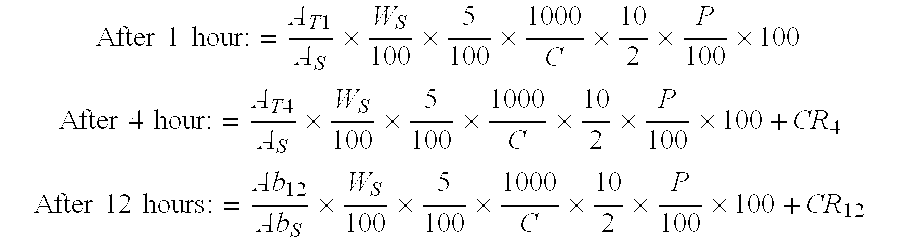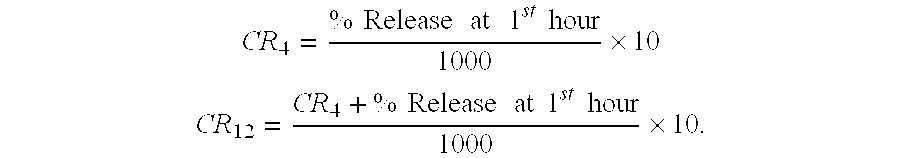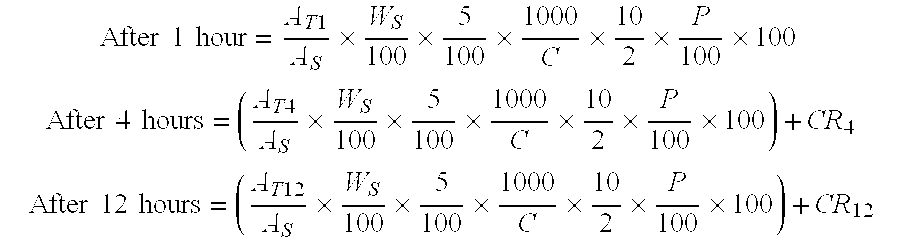Patents
Literature
51 results about "Sodium lauryl sulphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Metal alloy electroplating liquid
The invention discloses a metal alloy electroplating liquid. The metal alloy electroplating liquid is composed of the following raw material components in parts: 30-40 parts of copper sulphate, 30-50 parts of aluminium sulphate, 50-60 parts of copper chloride, 50-60 parts of nickel hydroxide, 20-30 parts of cobalt sulphate, 30-40 parts of cobalt chloride, 10-15 parts of iron sulphate, 30-35 parts of ferrous chloride, 50-58 parts of cuprous oxide, 16-18 parts of hydrochloric acid, 15-20 parts of acetic acid, 3-4 parts of Tween-60, 4-5 parts of sodium hydroxyethyl sulfonate, 5-6 parts of allyl polyethenoxy ether, 6-7 parts of polyethylene glycol, 3-4 parts of ammonium lauryl sulphate, 5-8 parts of sodium lauryl sulphate and 200-300 parts of deionized water. The metal alloy electroplating liquid is good in stability, the surface of a plated film is high in brightness and smoothness, the flexibility of a plated layer is obviously improved, and the defects of burrs, roughness and the like on the outer surface of a plated part are basically eliminated.
Owner:梁胜光
Method for preparing polybutadiene rubber latex with extra large particle size
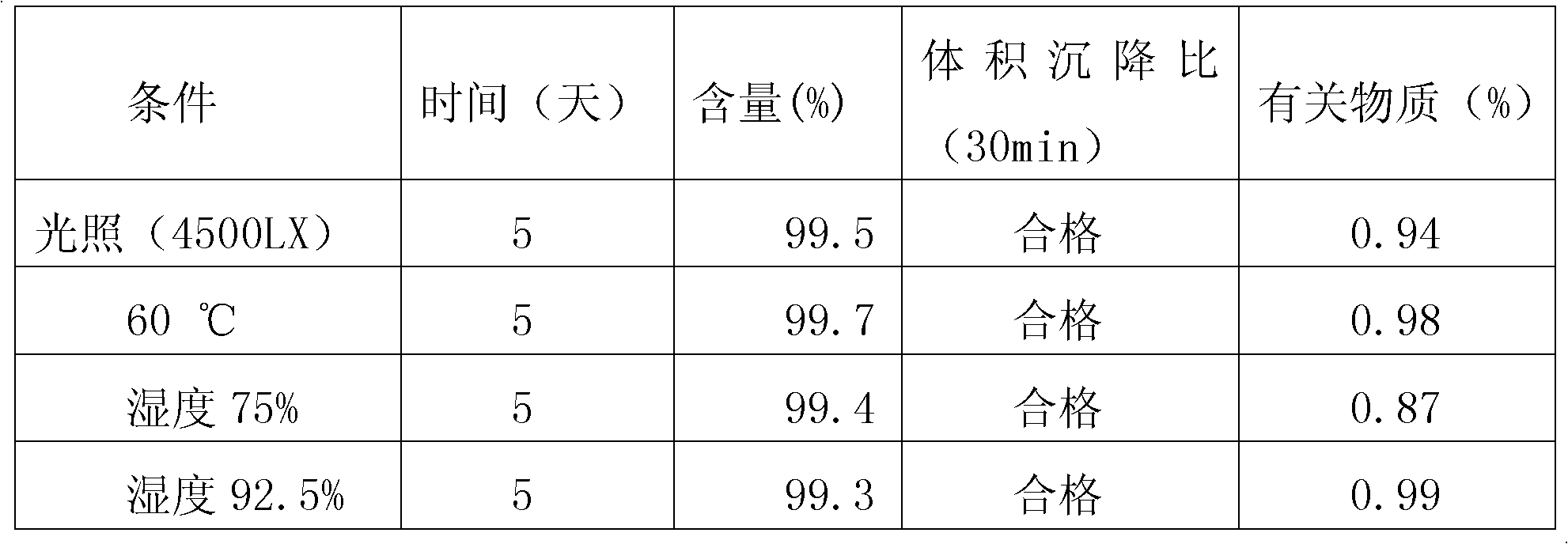
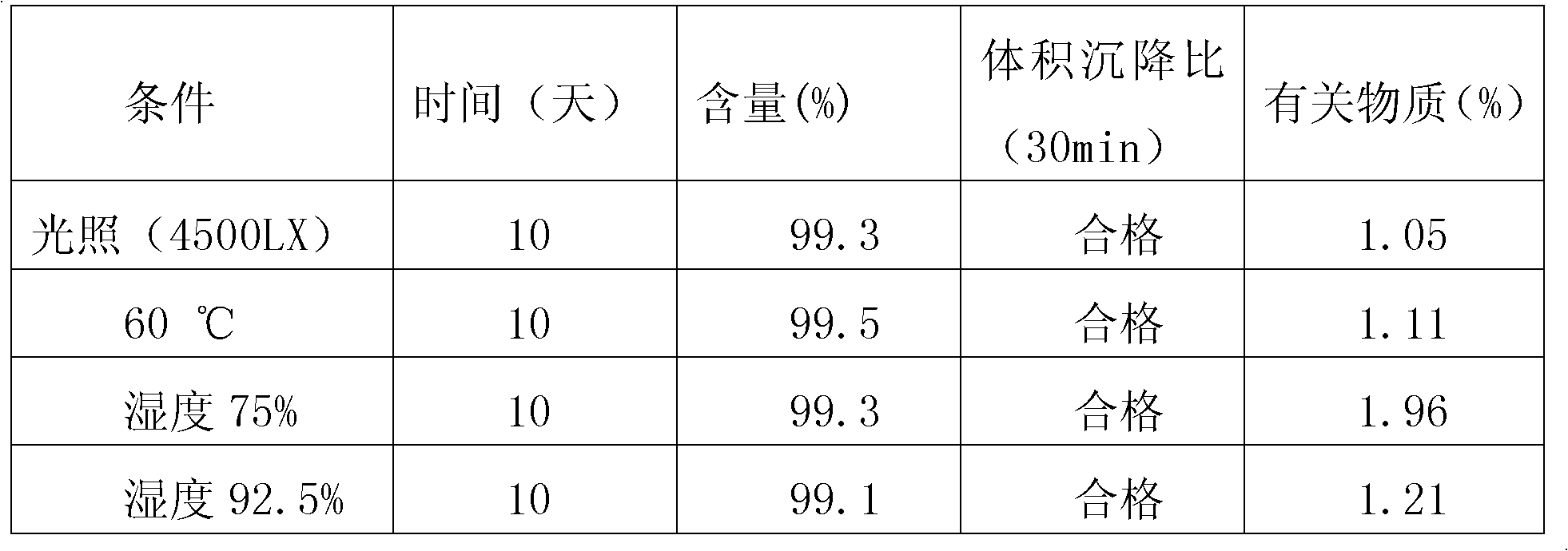
ActiveCN102050889ASimple preparation processMild conditions for agglomerationSodium bicarbonatePolymer science
The invention relates to a method for preparing polybutadiene rubber latex with extra large particle size, comprising the following steps: adding deionized water, sodium bicarbonate, sodium lauryl sulphate, potassium peroxydisulfate, dodecyl hydrosulfide, butyl acrylate and styrene into a reactor, carrying out replacement with nitrogen after stirring and emulsifying are started, heating, controlling the temperature to be 50-70 DEG C, polymerizing for 0.5-1 hour, then dropwise adding mixed monomers of styrene, methacrylic acid, butyl acrylate and OP-10, reacting for 1-4 hours, heating until the temperature is 70-85 DEG C, then stirring for 0.5-2 hours until the reaction is finished, and cooling and filtering to obtain a high polymer agglomerant; adding the high polymer agglomerant into polybutadiene rubber latex while slowly stirring for 1-60 minutes, and standing for 4 hours, thus the polybutadiene rubber latex with extra large particle size of 400-1000nm is obtained. The preparation process is simple, the agglomeration condition is mild, the agglomeration process is controllable, and the impact strength of an ABS (acrylonitrile butadiene styrene) finished product is greatly improved.
Owner:PETROCHINA CO LTD
Arbidol dry suspension and preparation method thereof
ActiveCN102000030AImprove complianceMask bitternessOrganic active ingredientsAntiviralsSodium cyclamateSodium sulfate
The invention discloses an arbidol dry suspension and a preparation method thereof, and belongs to the medical industry. The arbidol dry suspension comprises Arbidol hydrochloride, a suspending aid, a diluent, a lubricating agent, a flavoring agent and a pH regulator, wherein the suspending aid is one or more of Arabic gum, tragacanth, sodium alga acid, povidone, hydroxy propyl cellulose and xanthan gum; the diluent is saccharose, mannitol and microcrystalline cellulose; the lubricating agent is lauryl sodium sulfate and sodium lauryl sulphate; the flavoring agent comprises a sweetener and an aromatizer, the sweetener is mannitol, saccharose, sodium cyclamate and aspartame, and the aromatizer is orange essence, banana essence, strawberry essence, and pineapple essence; and the pH regulator is citric acid and tartaric acid. The preparation method comprises the following steps of: sieving the arbidol hydrochloride, and respectively crushing and sieving the suspending aid, the diluent, and the lubricating agent; fully mixing the components except the diluent, and then adding the diluent for uniform mixing; and wetting by using ethanol to prepare a soft material, drying, and packaging into an aluminum-plastic composite membrane bag. After the arbidol is prepared into the dry suspension, the bitter of the arbidol is effectively masked, and the compliance of the patient is greatly improved.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Mouth spray for preventing and treating nausea and emesis after tumor chemotherapy and radiotheraphy and preparation method thereof
InactiveCN101385712AEasy to increase or stop doseReduce efficacyAerosol deliveryPharmaceutical non-active ingredientsDiseaseAdditive ingredient
The invention relates to an oral spray for controlling nausea and vomit of chemotherapy and radiation therapy, the formula of the oral spray is composed of ingredients with the following parts by weight: 5-50 parts of drug absorption enhancer, 2-20 parts of drug active ingredient and 30-90 parts of buffer, the drug absorption enhancer can be any one or the combination of more of the following ingredients: azone, propylene glycol, polysorbate (Tween), ethylene glycol deoxycholic acid sodium salt, brij, sodium decanoate, lauric acid, stearic acid, sodium lauryl sulphate, stearyl alcohol sodium sulfate, dioctyl succinate sodium sulfonate, oleic acid, GK2, menthol and borneol; the drug active ingredient can be any one combination of the following ingredients: palonosetron hydrochloride, granisetron, ondansetron, azasetron and tropisetron; and the ingredients of the buffer are sodium citrate buffer solution and phosphate buffer solution. The oral spray provides a formulation which is safer, painless and convenient for patients with advanced tumor, elderly and weak patients, children patients and the patients who suffer from the metal illness and do not obey the oral administration or the injection drug administration.
Owner:陆飚 +1
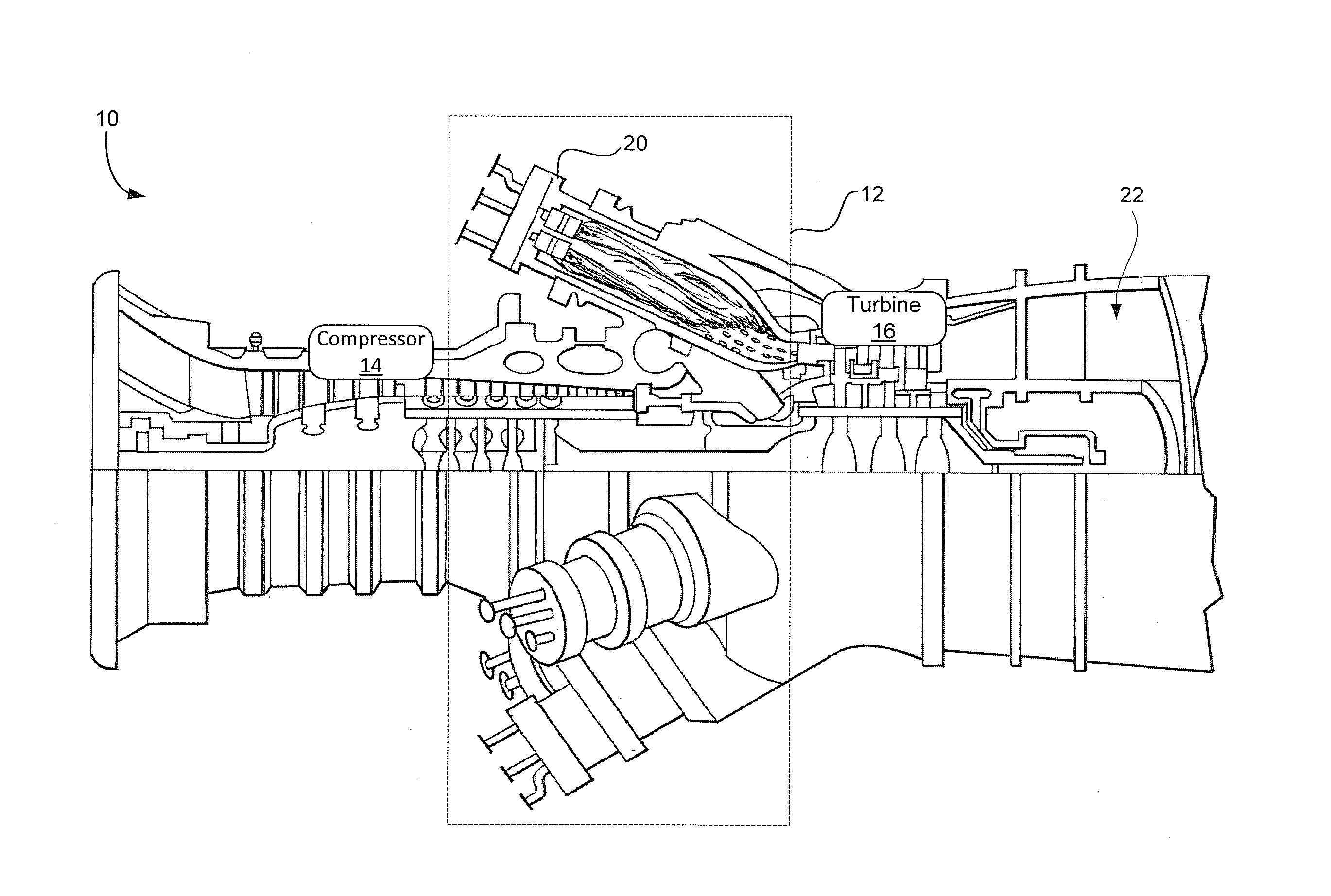
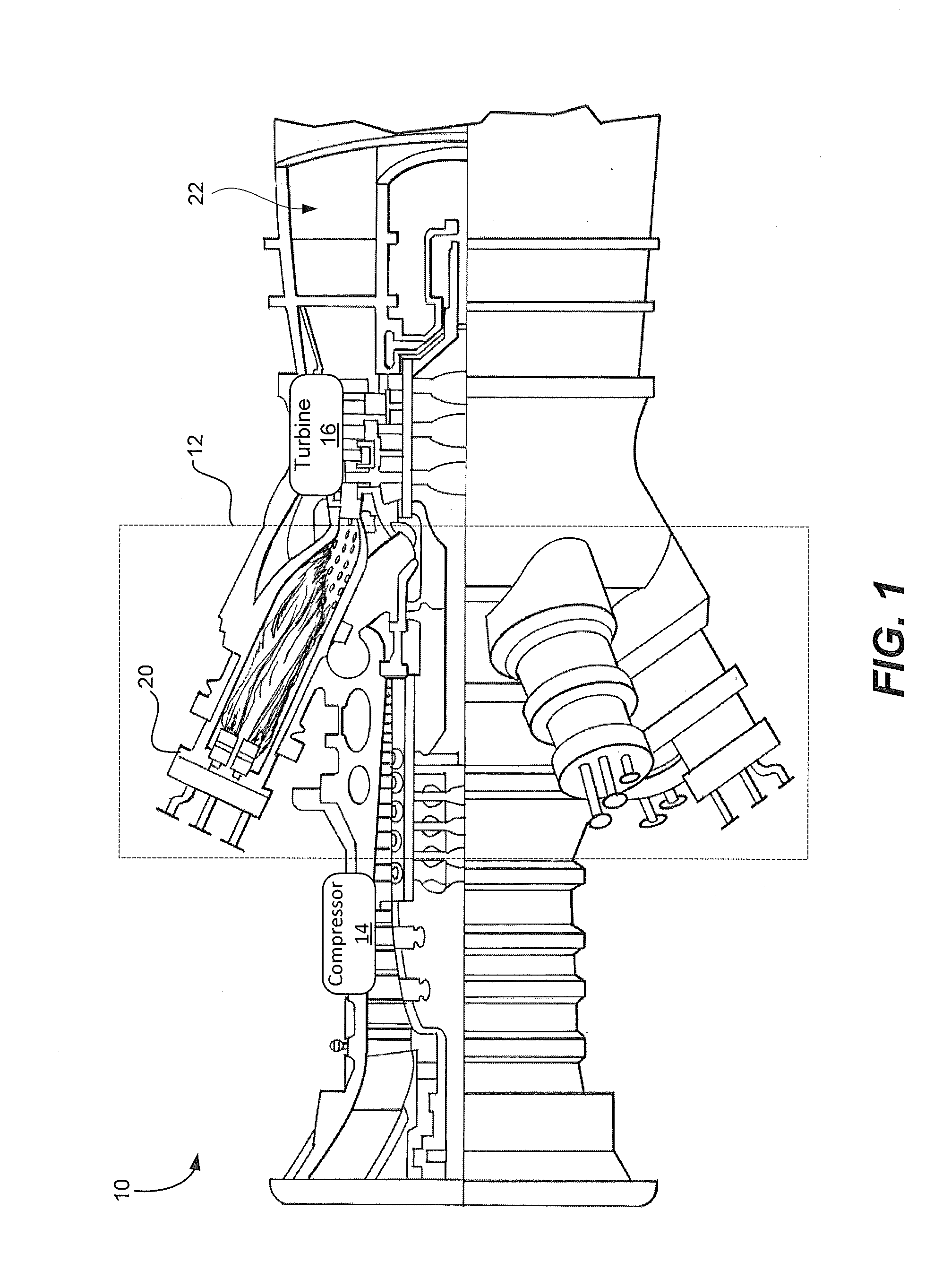
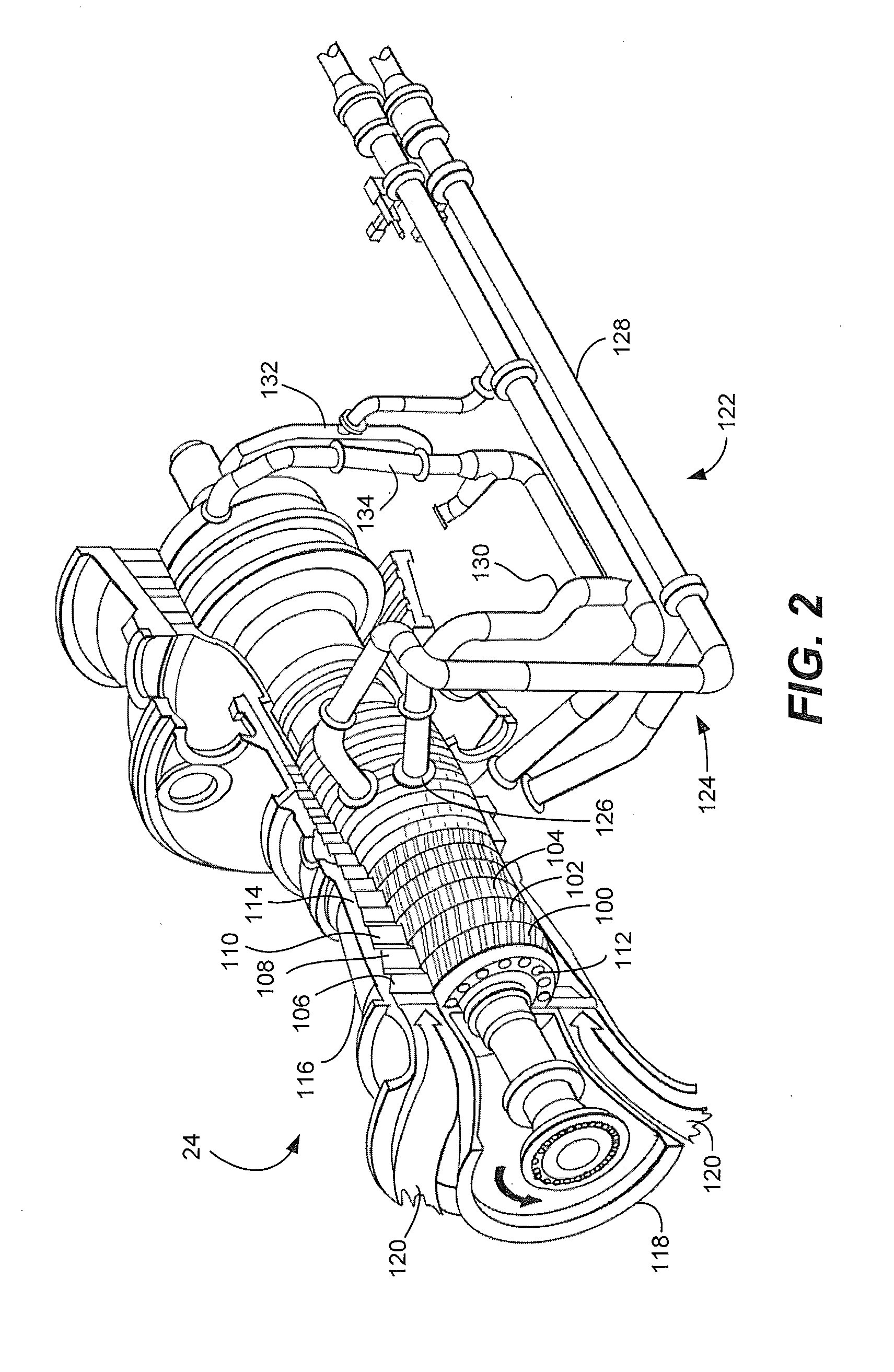
System and method for providing a wash treatment to a surface
Methods and systems for washing a surface, such as a gas turbine surface, are provided. A wash control system includes a storage tank configured to contain a cleaning agent, a plurality of nozzles, and a supply conduit coupled to the storage tank on a first end and the plurality of nozzles on a second end, wherein the wash control system is configured to deliver the cleaning agent from the storage tank and to discharge the cleaning agent through the plurality of nozzles and the cleaning agent includes an ethylene oxide-propylene oxide block copolymer, sodium dodecyl benzene sulphonate, sodium lauryl sulphate, or a combination including at least one of the foregoing.
Owner:GENERAL ELECTRIC CO
Liquid laundry detergent
InactiveCN103789104ASo as not to damageImprove the bactericidal effectAmpholytes/electroneutral surface-active compoundsDetergent compounding agentsBetaineCoconut diethanolamide
The present invention discloses a liquid laundry detergent, which comprises, by weight, 2-15 parts of sodium alcohol ether sulphate, 1-10 parts of sodium lauryl sulphate, 1-12 parts of coconut diethanolamide, 2-15 parts of dodecyldimethyl betaine, 1-13 parts of sorbitol fatty acid ester, 0.5-10 parts of a skin care extract, 0.1-5 parts of a thickening agent, 0.1-3 parts of a sterilization agent, and 40-91 parts of water, wherein the skin care extract is a mixture of olive oil, a cucumber extraction solution and a kumquat extraction solution, and the sterilization agent is a agrimonia pilosa extract. According to the product, the skin care extract and the sterilization agent adopting the Chinese herb agrimonia pilosa as the component are added in the formula, and the components are nature and mild, such that clothing can be effectively protected from damage during washing, the good sterilization and antibacterial effect is provided, and characteristics of safety and environmental protection are provided.
Owner:张释文
Starch-based wood adhesive and preparation method thereof
InactiveCN104946176AHigh bonding strengthImprove water resistanceNon-macromolecular adhesive additivesGraft polymer adhesivesAdhesiveCarvacryl acetate
The invention discloses a starch-based wood adhesive and a preparation method thereof. The starch-based wood adhesive is prepared by 30-40 parts of starch, 8-15 parts of fructose, 5-8 parts of butyl acrylate, 2-5 parts of vinyl acetate, 1-4 parts of urea, 3-5 parts of acetone, 2-4 parts of ethyl acrylate, 1-3 parts of isocyanate, 1-4 parts of sodium hydroxide, 1-3 parts of methyl isobutyl ketone, 1-3 parts of dioctyl phthalate, 2-4 parts of furfural, 1-3 parts of sodium lauryl sulphate, 1-2 parts of stabilizer, 1-2.2 parts of dispersing agents and 40-60 parts of water. The bonding strength, water resistant performance and stable storage performance of the adhesive are improved by taking the abundant and reproducible starch as the raw materials and through the property modification methods such as crosslinking and graft copolymerization.
Owner:SUZHOU YOUJUN ENVIRONMENTAL SCI & TECH
Preparation method of polybutylcyanoacrylate nanowire
The invention discloses a preparation method of a polybutylcyanoacrylate nanowire. The preparation method comprises the following steps of: preparing an oil-phase mixture, namely dispersing alpha-n butyl cyanoacrylate with the volume ratio of 0.2-0.3% and ethyl acetate with the volume ratio of 3-6% in acetone; preparing emulsified-phase mixture, namely dispersing a 5-8 g / L emulsifying agent and a 8-10 g / L stabilizer, wherein the emulsifying agent is sodium lauryl sulphate, and the stabilizer is dextran-70; carrying out interface emulsification polymerization reaction; and at room temperature, gradually dropwise adding the prepared oil-phase mixture into the prepared emulsified-phase mixture for carrying out interface emulsification polymerization. The preparation method disclosed by the invention has the characteristics of stable performance and no toxicity and no harm to a human body, animals and microorganisms.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Cyanogen-free leucocyte tri-grouping environment protection type haemolysin for blood cell analysis
InactiveCN101329229AEasy to measureAccurate concentrationPreparing sample for investigationBiological testingMedicine.hematologyHemoglobin formation
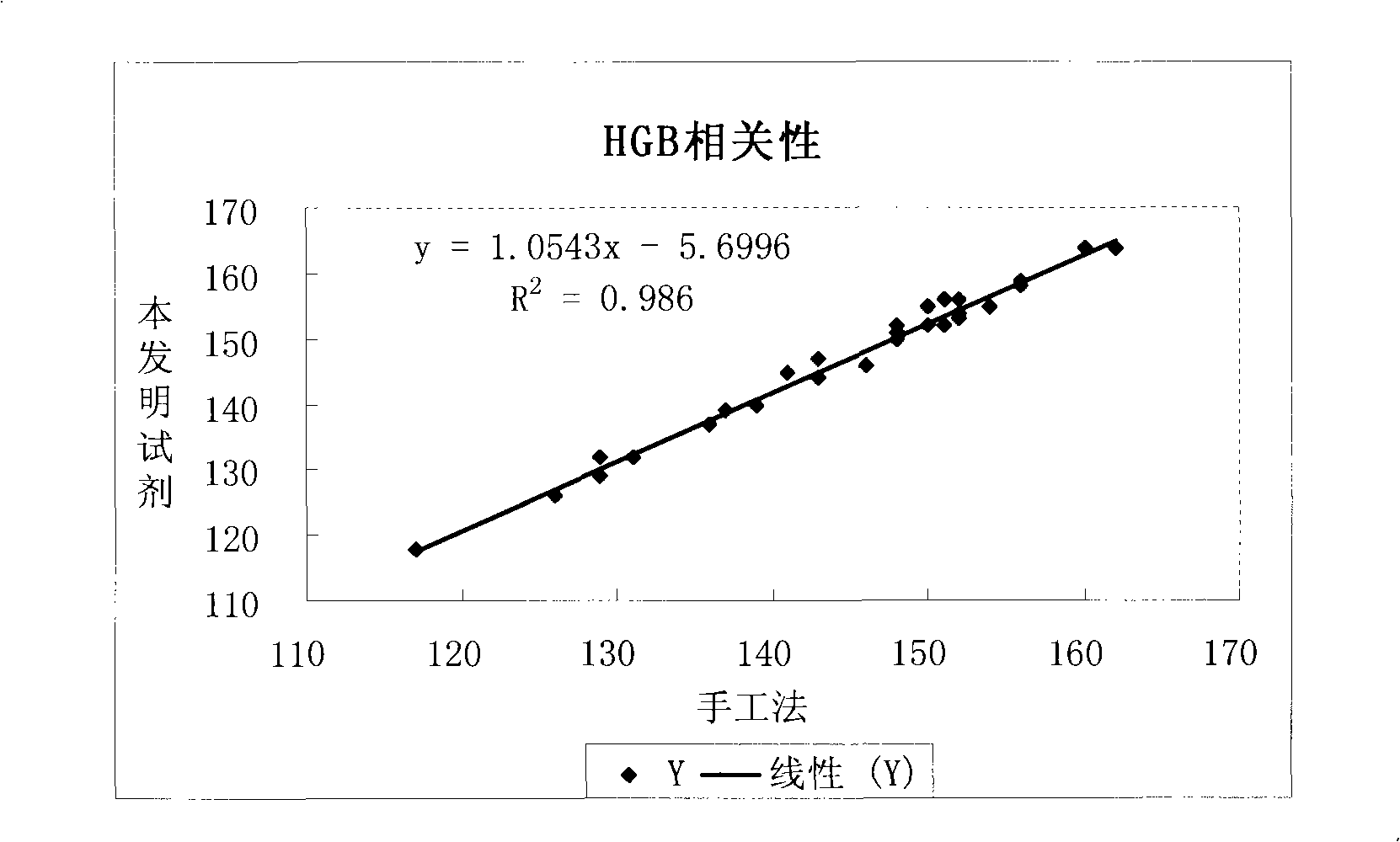
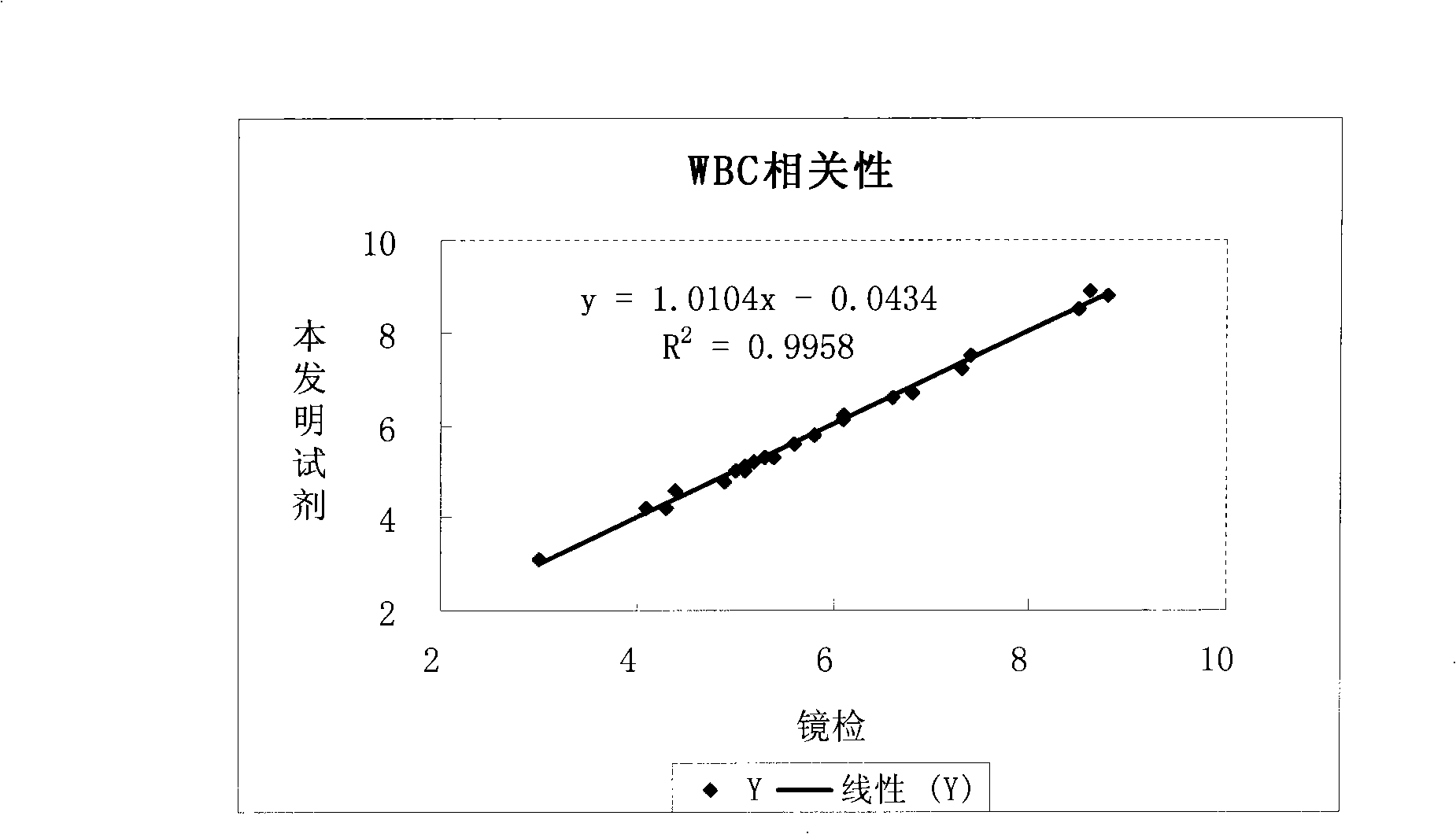
The invention relates to hemolysin used for blood cell analysis, in particular to the environmental-protection typed hemolysin of no-cyanogen leucocyte three-classification used for blood cell analysis. The hemolysin comprises the components as follows (by density): 0.5-5.0g / L of sodium lauryl sulphate (SLS), 3.0-10.0g / L of quaternary ammonium salt, 0.5-5.0g / L of nonionic surfactant, 0.5-3.0g / L of alkali metal salt, and residual quantity of deionized water. The hemolysin of the invention can quickly dissolve erythrocyte and release haemoglobin; the hemolysin and the haemoglobin form stable haemoglobin derivatives so as to lead the concentration to be exact and easy to be measured; furthermore, the leucocyte three-classification measurement is carried out simultaneously. The hemolysin of the invention can be highly relative to the HiCN mensuration recommended by International Council for Standardization in Hematology (ICSH) and can carry out the leucocyte three-classification exactly and quickly.
Owner:山东兰桥医学科技有限公司
Compound antioxidant for producing copper powder through water atomization method
ActiveCN104525959AImprove antioxidant capacityAddressing Higher Oxygen IssuesAntioxidantBenzotriazole
The invention discloses a compound antioxidant for producing copper powder through a water atomization method. The compound antioxidant comprises, by weight, 1-10 parts of benzotriazole, 1-10 parts of benzene sulfonic acid sodium salt, 1-10 parts of sodium lauryl sulphate, 2-10 parts of OP surfactants, 3-25 parts of glycerinum and 20-50 parts of water. According to the compound antioxidant for producing the copper powder through the water atomization method, by means of the component formula, the matching ration is reasonable, in the process of preparing the copper powder through the water atomization method, the free radical is complemented while film forming is conducted on the surface of the copper powder, the double-protection function is achieved, the good antioxidant effect is achieved, the antioxidant ability of the copper powder is improved effectively, and the problem that the oxygen content of copper power produced in a water atomization mode is higher is solved; in the process that the copper power is produced through the water atomization method, the antioxidant is added, the oxygen content of the copper powder produced through the water atomization method is lower than 0.08%, the oxygen content meets the national standard, the problem that the oxygen content of the copper powder obtained after water atomization is conducted is higher is solved, and the shelf life of the copper powder is prolonged.
Owner:佛山市顺德区美硕金属表面技术有限公司
Enteric-coated gelatine empty capsules and preparation method thereof
ActiveCN104434870APleasant fruity scentResidue reductionCapsule deliveryMacromolecular non-active ingredientsSolvent evaporationAcrylic resin
The invention discloses enteric-coated gelatine empty capsules. The enteric-coated gelatine empty capsules comprise the following components in parts by weight: 100 parts by weight of gelatine, 3-8 parts by weight of water, 8-15 parts by weight of sodium alginate, 8-15 parts by weight of acrylic resin, 20-45 parts by weight of n-butyl acetate, 5-25 parts by weight of sodium lauryl sulphate and 0-8 parts by weight of colorant. The invention further discloses a preparation method of the enteric-coated gelatine empty capsules. The preparation method disclosed by the invention is capable of effectively solving the technical problems of solvent evaporation during a forming process, resource waste, cost increase and air pollution, thus the capsule shell performance of the produced capsules is more excellent, and the pass percent of finished products is greatly increased.
Owner:SHAOXING KANGKE CAPSULE
AEtretin.bakthane water dispersible powder
InactiveCN101317576ANo pollution in the processChange limitationsBiocideAnimal repellantsWater dispersibleBacillus thuringiensis
The invention relates to a bio-pesticide which mainly solves the problems of the currently used bacillus thuringiensis (BT) such as unstable performance, narrow insecticidal spectrum and slow effect. The main compositions of the invention are avermectin and BT raw pesticide. The compositions also comprise filler calcium carbonate, detergent (LS), dispersant, white carbon black, sodium lauryl sulfate (K12) and solvent acetone. According to the weight percentage, the compositions comprise 0.2%-0.8% of avermectin, 5%-15% of BT, 2%-8% of detergent (LS), 2.8%-4% of dispersant (NNO), 3.5%-6% of white carbon black, 1%-5% of sodium lauryl sulphate (K12), 1%-3% of solvent acetone and 80%-85% of filler calcium carbonate. The bio-pesticide of the invention is a wettable powder and has the advantages of stable performance, good effect, wide insecticidal spectrum, quick effect, no effects on the environment, which is applicable for harmless and green agricultural products, forest and garden flowers.
Owner:周乃康 +1
Production method of PLA foam sheet
The invention provides a production method of a PLA (polylactic acid) foamed sheet, and the production method comprises the following steps of: pouring mixture formed by mixing 2-4% of nucleating agent, 1-3% of compound heat stabilizer, 0.3-0.5% of lubricating agent, 0.6-1% of dispersant and 1-4% of chain extender in mass ratio into 78.5-85.1% of a PLA material, mixing to be uniform, and conveying and melting by virtue of an extruder, wherein the nucleating agent is 3000-mesh talcum powder, the compound heat stabilizer is formed by mixing 80% of zinc stearate and 20% of calcium stearate in mass ratio, the lubricating agent is monoglyceride, the dispersant is sodium lauryl sulphate, and the chain extender is glycerine; then injecting 9% of butane with the purity of more than 98% at the pressure of 10-15 MPa to form a mixture at the temperature controlled to be 100-200 DEG C, and conveying the mixture to a moulding nose after being pressurized, mixed and cooled by virtue of a screw so as to be moulded into a foamed product. According to the invention, the PLA foamed sheet can be stably and reliably produced, the produced product has high foam density, small foam size, light mass and easiness in moulding, can substitute for most packaging material for food on the market and can be biologically degraded in nature, thus white pollution caused by the traditional macromolecule waste material to the environment can be greatly alleviated.
Owner:张建群
Anti-bacterial agents for treating childhood infections and infectious diseases and preparing method thereof
InactiveCN102379890AUnified specificationsEasy to carry and useAntibacterial agentsOrganic active ingredientsSucroseAntimicrobial drug
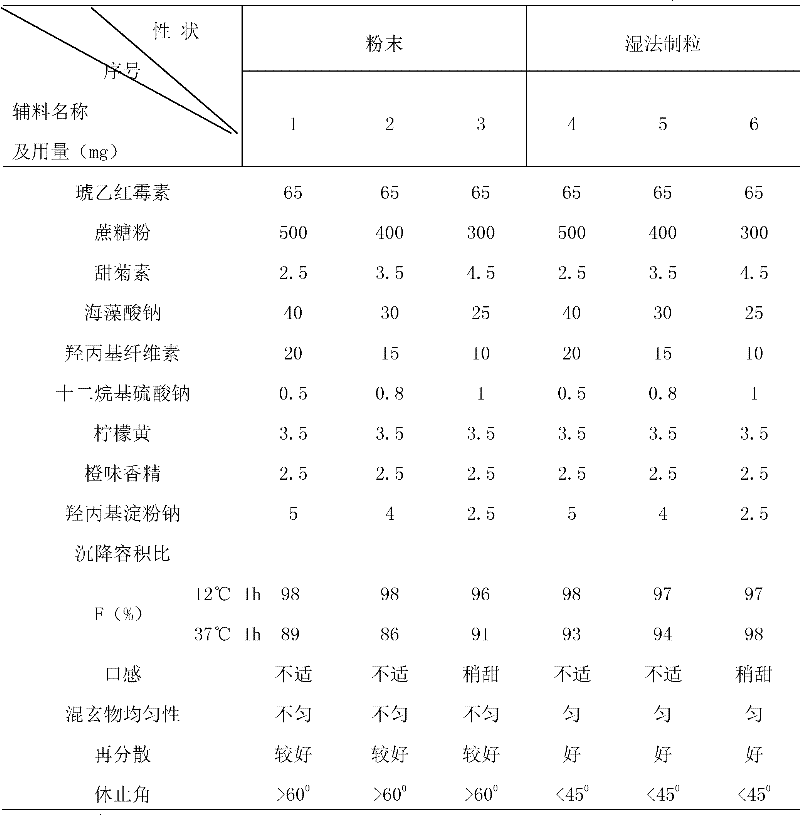
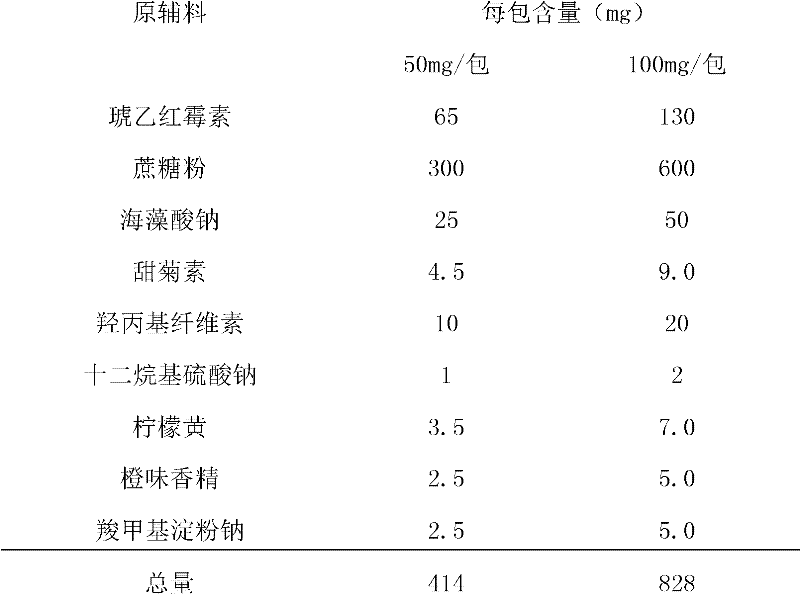
The invention relates to anti-bacterial agents for treating childhood infections and infectious diseases, which adopt erythromycin ethylsuccinate, sucrose powder, sodium alginate, steviosin, hydroxy propyl cellulose, sodium lauryl sulphate, tartrazine, orange essence and sodium carboxymethyl starch as raw materials. The preparation method comprises the following steps: 1) firstly screening the erythromycin ethylsuccinate, the sucrose powder and the steviosin, then mixing uniformly, later adding the sodium alginate, the hydroxy propyl cellulose, the sodium lauryl sulphate, the tartrazine and the sodium carboxymethyl starch in turn, then uniformly mixing and crushing as fine powder for further use; 2) adding the fine power obtained in the step 1) to a right amount of dilute ethanol liquor, then fully stirring to obtain soft material, and screening by a 30 meshes sieve to obtain wet particles; 3) drying the wet particles at 45 degrees centigrade, pelletizing by the 30 meshes sieve, adding the orange essence, uniformly mixing and packaging to prepare the dry suspension. The invention has unified specification, easily grasps the dosage, dose not limit the water quantity, can be conveniently carried and used, can be applied to children tastes and has obvious curative effects.
Owner:山西皇城相府药业股份有限公司
Externally applied emplastrum for treating anxious chronic bronchitis and preparation method thereof
ActiveCN102028927AImprove permeabilityDifficulty enhancing medicationRespiratory disorderOil/fats/waxes non-active ingredientsGlycerolOil phase
The invention relates to an externally applied emplastrum for treating anxious chronic bronchitis and a preparation method thereof and belongs to the technical field of externally applied medical emplastrum. The preparation method thereof comprises the following steps of: extracting the ephedra, asarum, pinellia ternate, rhizoma arisaematis, fructus gleditsiae, rhizoma dioscoreae nipponicae, grass leaf sweetflag rhizome, blackberry lily, scutellaria baicalensis and platycodon grandiflorum by refluxing ethanol, reducing pressure and recovering the ethanol, concentrating the extracted solution into extractum, mixing the extractum with the aqueous phase solution prepared from stearic acid, solid paraffin, wool oil and ethyl p-hydroxybenzoate and the oil phase solution prepared from glycerol, sodium lauryl sulphate and triethanolamine so as to form ointment, and then paving on a frame. The medicine can quickly permeate into pained part through the conduction of acupuncture point and meridians and collaterals and the percutaneous absorption, so as to get through the Ren and Du meridians, relieve cough and asthma and regulate qi-flowing for eliminating phlegm. The invention solves the problems that the western medicine cures the symptoms but not root causes by using, the treatment limitation is big, the orally-taken dosage for Chinese traditional treatment is big, the taking is inconvenient and the curative effect is slow. The emplastrum of the invention is convenient to use, has good curative effect, becomes effective quickly, is free from side effect and is fit for both children and adults.
Owner:杨福海
Local chicken farm sterilization disinfectant and preparation method of local chicken farm sterilization disinfectant
InactiveCN105165920AEnsure cleanliness and sterilitySimple preparation processBiocideFungicidesDiseaseAnimal science
The invention provides local chicken farm sterilization disinfectant, and relates to the technical field of livestock breeding. The local chicken farm sterilization disinfectant is prepared from the following ingredients including 3 to 5 parts of alkylolamides, 2 to 4 parts of silicon dioxide, 3 to 5 parts of potassium permanganate, 2 to 4 parts of polyaluminium chloride, 6 to 8 parts of coco-nut oil fatty acid monoethanol amine, 6 to 8 parts of sodium lauryl sulphate, 2 to 4 parts of wire drawing powder, 6 to 8 parts of clove juice, 6 to 8 parts of radix sophorae falvescentis liquid, 3 to 5 parts of oriental bittersweet powder, 8 to 10 parts of wild chrysanthemum flower liquid, 2 to 4 parts of eucalyptus oil and 60 to 80 parts of deionized water. The local chicken farm sterilization disinfectant has the beneficial effects that raw materials are rich; the price is low; the manufacturing process is simple and convenient; traditional Chinese preparations with the sterilization effects are adopted to be matched with degerming ingredients to be used for children farm sterilization; cleanness and sterility of chicken houses are ensured; the occurrence of chicken diseases is fundamentally solved.
Owner:蚌埠龙达农业专业合作社
Boron nitride/silica powder compounded ceramic nozzle and production method thereof
The invention discloses a boron nitride / silica powder compounded ceramic nozzle. The boron nitride / silica powder compounded ceramic nozzle is characterized by being prepared from the following raw materials in parts by weight: 70-80 parts of boron nitride, 3-6 parts of potassium feldspar, 4.1-5.6 parts of calcite, 7-12 parts of radium stone, 6-9 parts of blast furnace slag, 20-35 parts of ultrafine high-purity alumina powder, 1-2 parts of silica powder, 1.2-2.6 parts of polyacrylonitrile fibres, 0.6-0.9 part of sodium lauryl sulphate, 0.2-0.4 part of manganese sulphate, 0.5-1.1 parts of calcium carbonate, 6-9 parts of methyl methacrylate, 4-7 parts of dibutyl phthalate, 3-4 parts of ethyl alcohol, 2-4 parts of auxiliaries and an appropriate amount of water. According to the boron nitride / silica powder compounded ceramic nozzle disclosed by the invention, boron nitride and silica powder and compositely added to raw materials, meanwhile, blast furnace slag, radium stone and the like are further added, thus wastes are effectively utilized, cost is saved, and the performance indexes of the product are improved; the ceramic nozzle prepared by the method disclosed by the invention is good in chemical resistance, good in wear resistance, anti-corrosion at a high temperature, and capable of completely meeting the performance requirements of ceramic nozzles.
Owner:乌海市以诺实业有限责任公司
Anti-chapping skincare cream and preparation method thereof
InactiveCN106511143AReasonable formulaEasy to prepareCosmetic preparationsToilet preparationsAllantoinPropyl hydroxybenzoate
The invention provides anti-chapping skincare cream. The anti-chapping skincare cream is formed by proportioning a plurality of selected skincare materials, and has a better anti-chapping effect. The invention also provides a preparation method for the anti-chapping skincare cream. The anti-chapping skincare cream comprises the following components in parts by weight: 190 to 205 parts of water, 8 to 15 parts of glycerine, 1 to 4 parts of sodium lauryl sulphate, 0.5 to 1.5 parts of allantoin, 1 to 3 parts of petrolatum, 0.8 to 1.5 parts of ganoderma extract, 10 to 16 parts of mineral oil, 20 to 26 parts of stearyl alcohol, 0.1 to 1 part of methylparaben, 0.1 to 1 part of propyl hydroxybenzoate, 0.1 to 0.8 part of glyceryl stearate and 0.5 to 1 part of essence.
Owner:无锡市汉方八珍科技有限公司
Novel pharmaceutical modified release dosage form composition comprising cyclooxygenase enzyme inhibitor
InactiveCN101227893AExtension of timeNo toxicityNervous disorderAntipyreticModified Release Dosage FormEster prodrug
Pharmaceutical modified release dosage form comprising at least one cyclooxygenase enzyme inhibitor or its pharmaceutically acceptable salts, esters, prodrugs, solvates, hydrates, or derivatives thereof as active agent, with a pharmaceutically acceptable carrier for controlling the release of the cyclooxygenase enzyme inhibitor is provided. The dosage form preferably provides a release of not more than about 60 % of the cyclooxygenase enzyme inhibitor in 1 hour and not less than about 75 % of the cyclooxygenase enzyme inhibitor after 12 hours when tested in accordance with the dissolution method (I) described herein employing Distilled water with 2.0 % Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (II) described herein employing pH 7.0 Phosphate buffer with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (III) described herein employing 0.001 N Hydrochloric acid with 1.0 % Sodium lauryl sulphate as dissolution medium. Further, the pharmaceutical composition of the present invention when tested in a group of healthy humans preferably achieves a mean peak plasma concentration (Cmax) after at least about 1 hour of administration of the dosage form,. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such dosage form.
Owner:PANACEA BIOTEC
Novel Pharmaceutical Modified Release Dosage Form Cyclooxygenase Enzyme Inhibitor
InactiveUS20100204333A1Easy and cost-effectiveBiocideNervous disorderModified Release Dosage FormPhosphate
Pharmaceutical modified release dosage form comprising at least one cyclooxygenase enzyme inhibitor or its pharmaceutically acceptable salts, esters, prodrugs, solvates, hydrates, or derivatives thereof as active agent, with a pharmaceutically acceptable carrier for controlling the release of the cyclooxygenase enzyme inhibitor is provided. The dosage form preferably provides a release of not more than about 60% of the cyclooxygenase enzyme inhibitor in 1 hour and not less than about 75% of the cyclooxygenase enzyme inhibitor after 12 hours when tested in accordance with the dissolution method (I) described herein employing Distilled water with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (II) described herein employing pH 7.0 Phosphate buffer with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (III) described herein employing 0.001 N Hydrochloric acid with 1.0% Sodium lauryl sulphate as dissolution medium. Further, the pharmaceutical composition of the present invention when tested in a group of healthy humans preferably achieves a mean peak plasma concentration (Cmax) after at least about 1 hour of administration of the dosage form. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such dosage form.
Owner:PANACEA BIOTEC
Solid surfactant composition
ActiveUS20140336095A1Reduce impactMaintain product qualityInorganic/elemental detergent compounding agentsAnionic surface-active compoundsLauryl ether sulfateSURFACTANT BLEND
A surfactant product is in the form of a solid. The surfactant product includes (i) sodium carbonate; (ii) cream of tartar; (iii) glycerine; (iv) sodium lauryl sulphate in an amount of from 3 to 15 wt % based on the weight of the total composition, and (v) sodium laureth sulphate in an amount of from 10 to 25 wt % based on the weight of the total composition.
Owner:COSMETIC WARRIORS
Azo compound dye and preparation method thereof
The invention discloses an azo composite dye, which comprises the following components in parts by mass: 80-100 parts of azobisisobutyronitrile, 30-40 parts of m-phenylenediamine, and 40-60 parts of dodecanol Sodium sulfate, 20~30 parts of monoglyceride, 10~30 parts of p-methylaniline, 10~30 parts of curing agent and 120~150 parts of water. At the same time, the invention also discloses a preparation method of the azo composite dye. Compared with the traditional technology, the present invention improves the stability of the azo composite dye by adding highly stable organic aromatic hydrocarbon groups and low-temperature modification treatment, and avoids the decomposition of various carcinogenic aromatic hydrocarbons due to environmental influences in the later stage.
Owner:无锡润新染料有限公司
Soybean-based protein adhesive and preparation method thereof
InactiveCN104946197AHigh bonding strengthImprove water resistanceNon-macromolecular adhesive additivesProtein adhesivesCelluloseVulcanization
The invention discloses a soybean-based protein adhesive and a preparation method thereof. The soybean-based protein adhesive is prepared by 20-30 parts of bean flour, 5-10 parts of polyhydric alcohols, 5-8 parts of sodium lauryl sulphate, 10-15 parts of trimethylolpropane triester, 2-6 parts of urea, 3-5 parts of carboxyethyl cellulose, 1-3 parts of vulcanization diethyl succinate, 1-2 part of isocyanate, 1-2 parts of sodium hydroxide, 1-3 parts of dibenzoyl peroxide, 1-3 parts of maleic anhydride, 1-2 parts of nitrite diisopropylamine, 1-2 parts of stabilizers and 40-60 parts of water. The bonding strength, water resistant performance and stable storage performance of the adhesive are improved by taking the abundant and reproducible bean flour as the raw materials and through the property modification methods such as crosslinking and graft copolymerization.
Owner:SUZHOU YOUJUN ENVIRONMENTAL SCI & TECH
Two-phase composition additive, application and preparation method of two-phase composition additive
InactiveCN107579290AImprove electrochemical activityGood effectFinal product manufactureSecondary cells servicing/maintenanceHardnessSilicon rubber
The invention provides a two-phase composition additive, application and a preparation method of the two-phase composition additive. The two-phase composition additive is prepared from 10 to 90 volumeparts of a siloxane composition and 10 to 90 volume parts of a silicon dioxide mixture; based on 100 volume parts, the siloxane composition comprises 2 to 12 volume parts of siloxane of which the main chain skeleton is of a (-Si-O-Si-) structure, 2 to 12 volume parts of organic silicon rubber and 76 to 96 volume parts of tetraethyl orthosilicate; based on 100 volume parts, the silicon dioxide mixture comprises 5 to 20 volume parts of fumed silica, 78 to 94.5 volume parts of industrial pure water, and 0.5 to 2 parts of sodium lauryl sulphate; the two-phase composition additive is prepared according to the process method provided by the invention, is used for mixing with sulphuric acid liquid and is added into a lead storage battery so as to enhance the softening resistance of positive active material and help a softened active material to recover hardness; the adding amount is small, and the cost performance is high.
Owner:东莞恒量新能源科技有限公司 +1
Waterproof adhesion with long quality guarantee period for paper product processing and preparation method of waterproof adhesion
InactiveCN107459958AImprove anti-fouling and anti-bacterial propertiesWell mixedNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesPhenyl EthersPolyvinyl alcohol
The invention discloses a waterproof adhesion with long quality guarantee period for paper product processing. The waterproof adhesion with long quality guarantee period for paper product processing comprises the following raw materials: polyurethane, polyvinyl alcohol, sodium lauryl sulphate, polyoxy ethylene nonyl phenyl ether, ammonium persulphate, dibutyl phthalate, 1,2-benzisothiazolinone, s-triazine, benzoxazoline, formaldehyde, urea, carboxymethyl cellulose, oxalic acid, calcium stearate, lime milk, aluminum sulfate, light calcium carbonate, bentonite, titanium dioxide, an emulsifying agent, a defoamer, a silane coupling agent KH-550, a pollution-resistant antibacterial auxiliary agent, a waterproof modified auxiliary agent and deionized water. The invention further provides a preparation method of the waterproof adhesion with long quality guarantee period for paper product processing. The prepared adhesive is used for paper product processing, and has excellent waterproof performance and long quality guarantee period.
Owner:ANHUI ANDA HUATAI NEW MATERIALS CO LTD
Dental ulcer ointment containing marine biological ingredients
InactiveCN103690599AEnsure balanceNatural ingredientsOrganic active ingredientsAerosol deliveryGlycerolAugmented Ointment
The invention discloses a dental ulcer ointment containing marine biological ingredients. The dental ulcer ointment comprises the following components in percentage by weight: 10-15% of albolene, 5-10% of liquid paraffin, 6-12% of hexadecanol, 3-8% of micro-sized hydroxyapatite, 5-8% of potassium nitrate, 0.5-1.5% of glycerol monostearate, 0.5-1.0% of sodium lauryl sulphate, 4-8% of glycerinum, 0.01-0.03% of ethylparaben, 5-6% of algal polysaccharides, 6-10% of honeysuckle extract solution, 15-20% of mint extract solution, and the balance of deionized water. The dental ulcer ointment containing marine biological ingredients disclosed by the invention has the transcendental effects of removing bacteria and diminishing inflammation and is capable of effectively preventing and treating dental inflammations, such as dental ulcer, swelling and aching of gum and the like; the components are natural, safe and effective.
Owner:QINGDAO HIFUN MARINE BIOLOGICAL TECH
Externally applied emplastrum for treating anxious chronic bronchitis and preparation method thereof
ActiveCN102028927BImprove permeabilityEase the difficulty of medicationRespiratory disorderOil/fats/waxes non-active ingredientsGlycerolOil phase
The invention relates to an externally applied emplastrum for treating anxious chronic bronchitis and a preparation method thereof and belongs to the technical field of externally applied medical emplastrum. The preparation method thereof comprises the following steps of: extracting the ephedra, asarum, pinellia ternate, rhizoma arisaematis, fructus gleditsiae, rhizoma dioscoreae nipponicae, grass leaf sweetflag rhizome, blackberry lily, scutellaria baicalensis and platycodon grandiflorum by refluxing ethanol, reducing pressure and recovering the ethanol, concentrating the extracted solution into extractum, mixing the extractum with the aqueous phase solution prepared from stearic acid, solid paraffin, wool oil and ethyl p-hydroxybenzoate and the oil phase solution prepared from glycerol, sodium lauryl sulphate and triethanolamine so as to form ointment, and then paving on a frame. The medicine can quickly permeate into pained part through the conduction of acupuncture point and meridians and collaterals and the percutaneous absorption, so as to get through the Ren and Du meridians, relieve cough and asthma and regulate qi-flowing for eliminating phlegm. The invention solves the problems that the western medicine cures the symptoms but not root causes by using, the treatment limitation is big, the orally-taken dosage for Chinese traditional treatment is big, the taking is inconvenient and the curative effect is slow. The emplastrum of the invention is convenient to use, has good curative effect, becomes effective quickly, is free from side effect and is fit for both children and adults.
Owner:杨福海
Acrylate coating for aircraft propeller blades
The invention discloses acrylate coating for aircraft propeller blades. The acrylate coating comprises the following raw materials in parts by weight: 70-80 parts of a main material, 1-4 parts of a core-shell toughening agent, 1-4 parts of a vermiculite powder, 2-4 parts of fly ash, 1-4 parts of graphene, 1-4 parts of calcined kaolin, 2-4 parts of active carbon, 1-3 parts of nonylphenol polyoxyethylene ether, 2-8 parts of vinyl triamine, 1-2 parts of a copper antifoulant, 1-2 parts of a thixotropic agent, 1-2 parts of a dispersing agent, 1-2 parts of a defoaming agent and 50-120 parts of deionized water, wherein the core-shell toughening agent is prepared by the following technology: mixing butyl acrylate, hydroxyethyl acrylate, sodium lauryl sulphate, ammonium persulfate and water, and heating while stirring to obtain a core layer emulsion; mixing vinyl chloride, hydroxypropyl acrylate, polyvinyl alcohol, benzoyl peroxide and water, and heating while stirring to obtain a shell layer emulsion; mixing the core layer emulsion and the shell layer emulsion, heating, dropwise adding an aqueous formaldehyde solution, continuously stirring after dropwise addition, cooling, and filtering to obtain the core-shell toughening agent.
Owner:安徽劲旋风航空科技有限公司
Fast cleaning fluid for mechanical equipment
The invention discloses a fast cleaning fluid for mechanical equipment. The fast cleaning fluid is prepared from the following raw materials in parts by weight: 6.2-8.4 parts of phyllosilicate, 5.2-8.7 parts of hexadecyl phosphate, 5.5-7.5 parts of alkyl polyglucoside, 2.8-4.5 parts of sodium lauryl sulphate, 4.2-5.3 parts of silicon oxide, 4.5-5.6 parts of non-phosphorus water-softening agents, 1.3-2.5 parts of glycerol and 1.8-4.5 parts of latent solvents. The cleaning agent for a metal part, which is disclosed by the invention, is high in infiltration capacity, can be infiltrated into the bottom layer of a cleaned substance, can be used for fast dissolving and removing various dirt and impurities which are attached to the surface of the metal part, does not generate the phenomenon of redeposition during cleaning, has no corrosion and no damage on the metal surface in a cleaning process, is high in cleaning speed, enables the metal surface to be clean, bright and good in quality after cleaning and can effectively ensure the machining accuracy of metal.
Owner:QINGDAO HUIERTONG TRADING
Agrochemical electrolyte compositions
An agrochemical concentrate comprises lecithin and at least one agrochemical active. The agrochemical concentrate may also comprise a dispersant selected from sucrose ester, alkylpolyglucoside, alkylnaphthalene sulphonates, phosphate ester, sorbitol ester, polyglycerol ester, alkyl sulphates, sodium lauryl sulphate, alkylglucamides, and dialkyl sulphosuccinates. An aqueous formulation comprises a dilution of the agrochemical concentrate in an electrolyte. A pre-blend comprises lecithin and a dispersant. A method of preparing the agrochemical concentrate and a method for treating vegetation are also disclosed.
Owner:CRODA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com