Orodispersible tablets of erythritol and isomalt
A technology of isomalt and erythritol, which is applied in the directions of pill delivery, medical preparations of non-active ingredients, and pharmaceutical formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The crude erythritol product (Cargill Zerose® 16957) was ground in a Bauermeister turbine mill UTL with a 1 mm sieve to obtain a powder with a volume mean diameter of 25 μm. The volume mean diameter is measured by laser diffraction.
[0071] 400 g of ground erythritol powder was mixed with 100 g of isomalt (Cargill C*lsoMaltidex ?) for 10 seconds of dry mixing.
[0072] Add 30ml of water dropwise at a rate of 5ml / min. After the liquid was added, the blend was continued to mix for 60 seconds.
[0073] The granulated powder was hand wet sieved with a 2 mm sieve.
[0074] The wet sieved granules were dried in a fluidized bed (Aeromatic-Fielder GEA-Strea-1) at a temperature of 60° C. for 30 minutes.
[0075] The dried granules were sieved through a 0.315 mm sieve for 5-10 minutes in a granulator (Erweka (FGS+AR400E)) at 100 rpm.
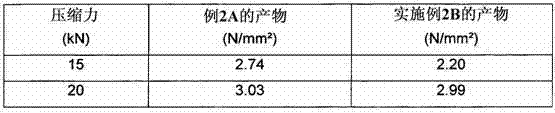
example 2A
[0076] Example 2A - Comparative Example
[0077] The granulated product obtained from Example 1 was then mixed with 1% magnesium stearate in a Pharmatech apparatus at a speed of 28 rpm.
[0078] The granulated product was compressed in a tablet press (Korsch-PH100) with a compression force varying from 5 kN to 20 kN.
[0079] Tablets have a 1cm 2 The surface of the tablet has a diameter of 11.3mm and its weight is 350mg.
Embodiment 2B
[0081] The granulated product obtained from Example 1 was then (dry) blended in a Pharmatech apparatus at 28 rpm with 2% Ac-di-sol (disintegrant) and 1% magnesium stearate.
[0082] The granulated product was compressed in a tablet press (Korsch-PH100) with a compression force varying from 5 kN to 20 kN.
[0083] Tablets have a 1cm 2 The surface of the tablet has a diameter of 11.3mm and its weight is 350mg.
[0084] Example 3 - Comparative example - according to WO2010 / 025796
[0085]The crude erythritol product (Cargill C*PharmEridex 16956) was ground in a Bauermeister turbine mill UTL with a 1 mm sieve to obtain a powder with a volume mean diameter of 30 μm.
[0086] The volume mean diameter is measured by laser diffraction. Erythritol has 0.40m 2 / g specific surface area.
[0087] 500 g of ground erythritol powder were dry blended for 60 seconds in a high shear mixer (Pro-C-ept-Mi-Pro, chopper: 3000 rpm, and impeller: 1200 rpm).
[0088] 79.17 g of liquid sorbitol (7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com