Synthesis technique for obtaining difluoro oxalate lithium borate and di-oxalate lithium borate
A technology of lithium difluorooxalate borate and lithium bisoxalate borate, which is applied in the field of synthetic technology for simultaneously obtaining lithium difluorooxalate borate and lithium bisoxalate borate, can solve the problems of limited research, high equipment requirements, unfavorable industrial production, difluorooxalate Lithium borate is developed late and other issues, to achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
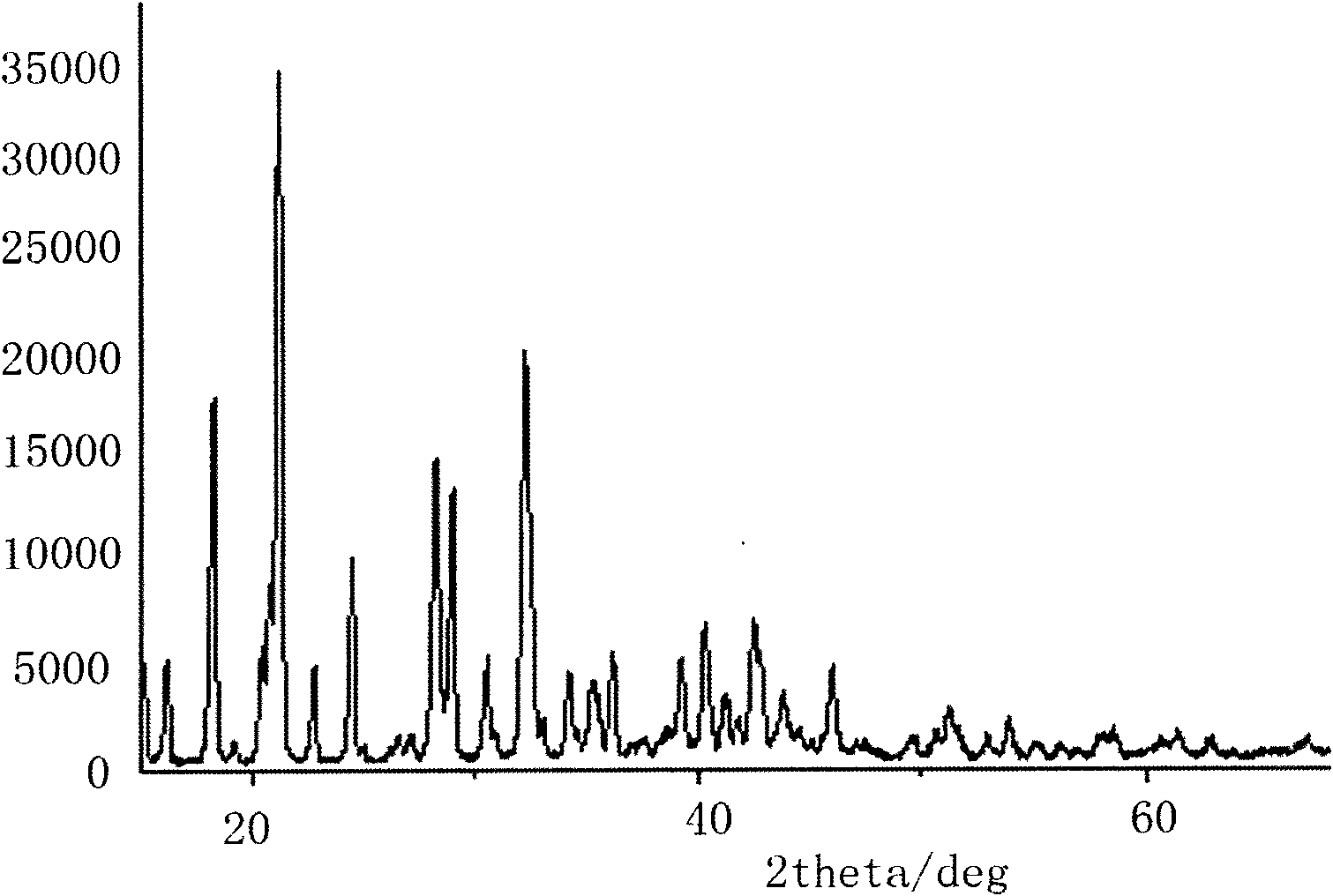
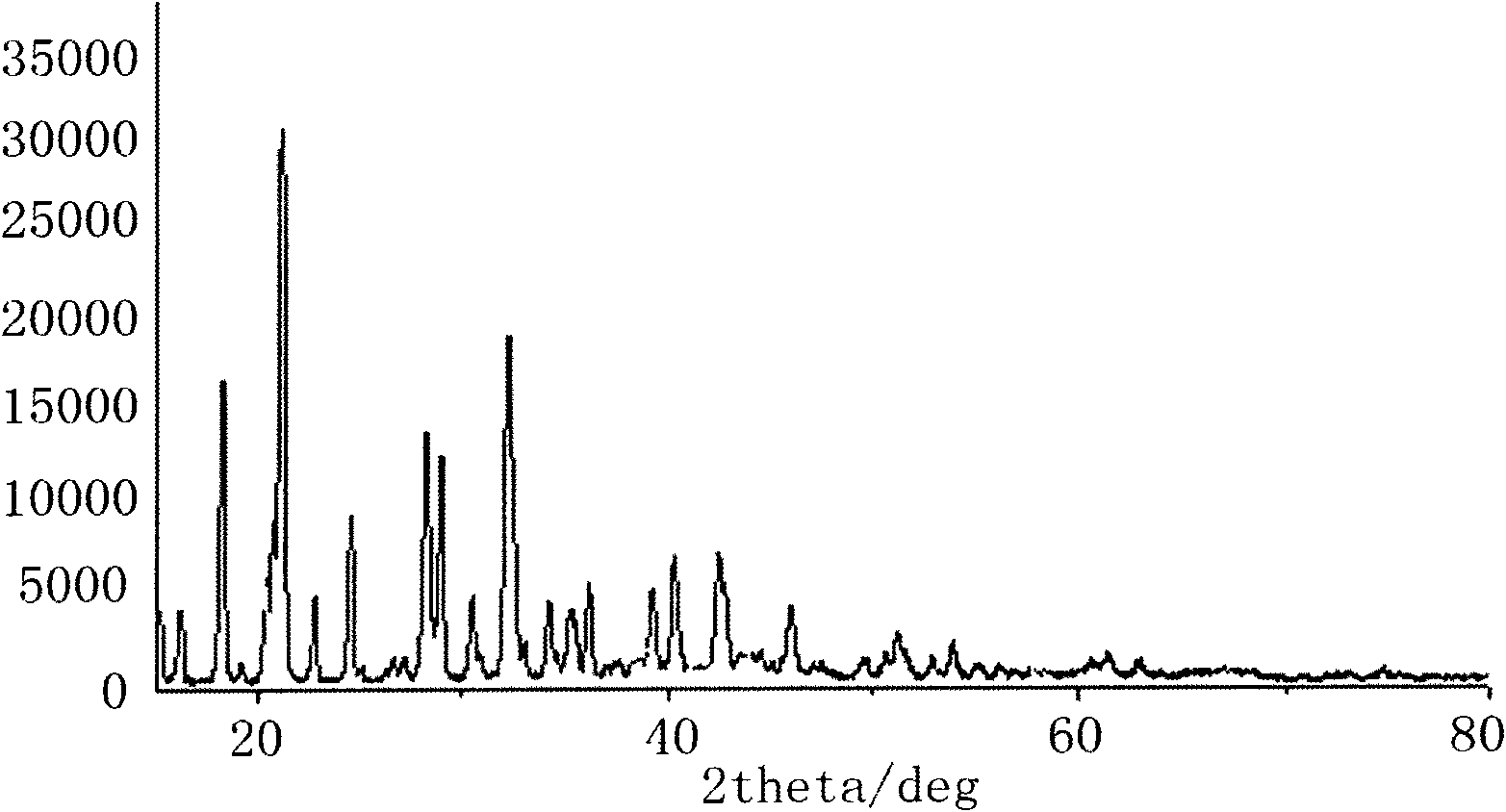
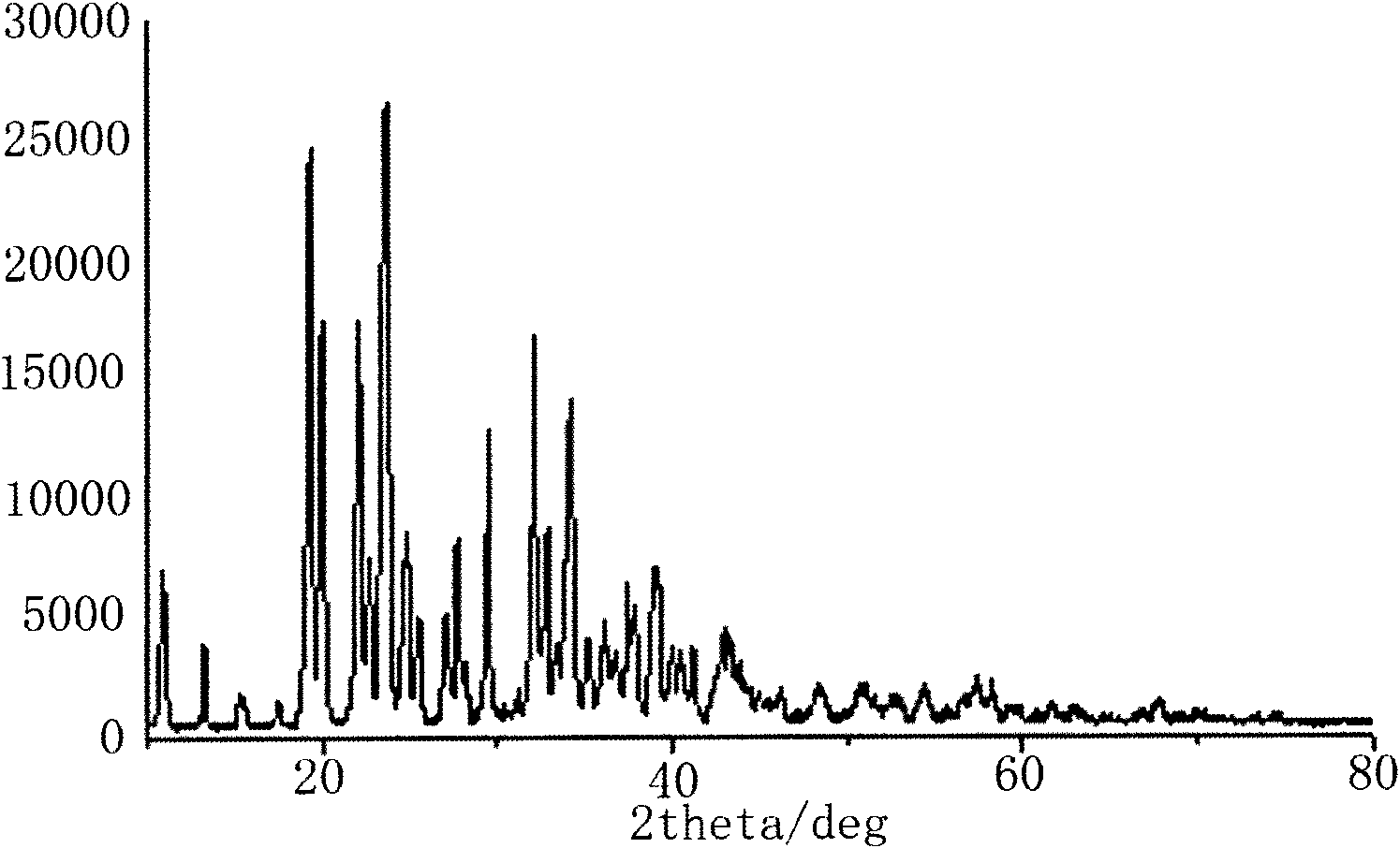
Image
Examples
Embodiment 1
[0024] Using boron trifluoride ether solution and lithium oxalate as raw materials to prepare lithium difluorooxalate borate and lithium bisoxalate borate.
[0025] Step 1. Add 101.9g of lithium oxalate dried at 150°C for 10h into a dry reaction vessel equipped with a magnetic stirrer and a thermometer, add 500g of dimethyl carbonate and stir, and slowly add 94.5g of boron trifluoride ether solution dropwise, while Heat and stir the reaction while adding dropwise, the addition is completed in 2 hours, the reaction temperature is adjusted to 80°C for 24 hours, and the reaction produces lithium difluorooxalate borate soluble in dimethyl carbonate and lithium bisoxalate borate insoluble in dimethyl carbonate and fluorine Lithium, then cooled to room temperature.
[0026] Step 2, filter the reacted mixture at normal temperature to remove unreacted lithium oxalate and lithium fluoride and lithium bisoxalate borate generated after the reaction, extract the lithium bisoxalate borate ...
Embodiment 2
[0030] Lithium difluorooxalate borate and lithium bisoxalate borate were prepared by using fluoboric acid, oxalic acid and lithium carbonate as raw materials.
[0031] Step 1. In a reactor equipped with a magnetic stirrer, add 136g of oxalic acid and 160g of fluoboric acid to 300g of acetonitrile and stir until the emulsion is dissolved, then slowly add 295g of lithium carbonate, and stir the reaction while adding. When no gas appeared, the reaction was continued for 5 hours to obtain lithium difluorooxalate borate, lithium bisoxalate borate solution and lithium fluoride.
[0032] Step 2, filter the reaction mixture at room temperature to remove unreacted lithium oxalate and lithium fluoride generated after the reaction, evaporate the solution to solids on a rotary evaporator, and extract the difluorine in the solids with diethyl carbonate several times Lithium oxalate borate, concentrated under reduced pressure, crystallized by cooling with diethyl carbonate, and recrystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com