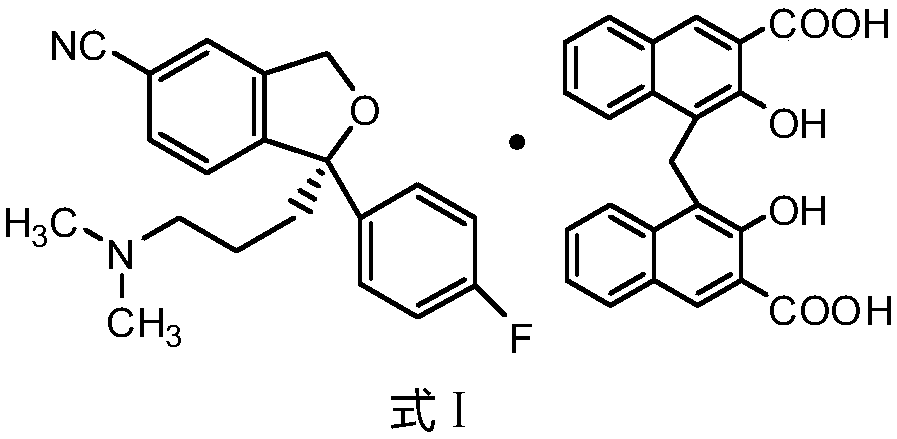
Novel preparation process of high-purity escitalopram pamoate
A technology of pamoate and oxalate, applied in the field of chemical medicine, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Implementation Example 1: Preparation of Escitalopram Pamoate
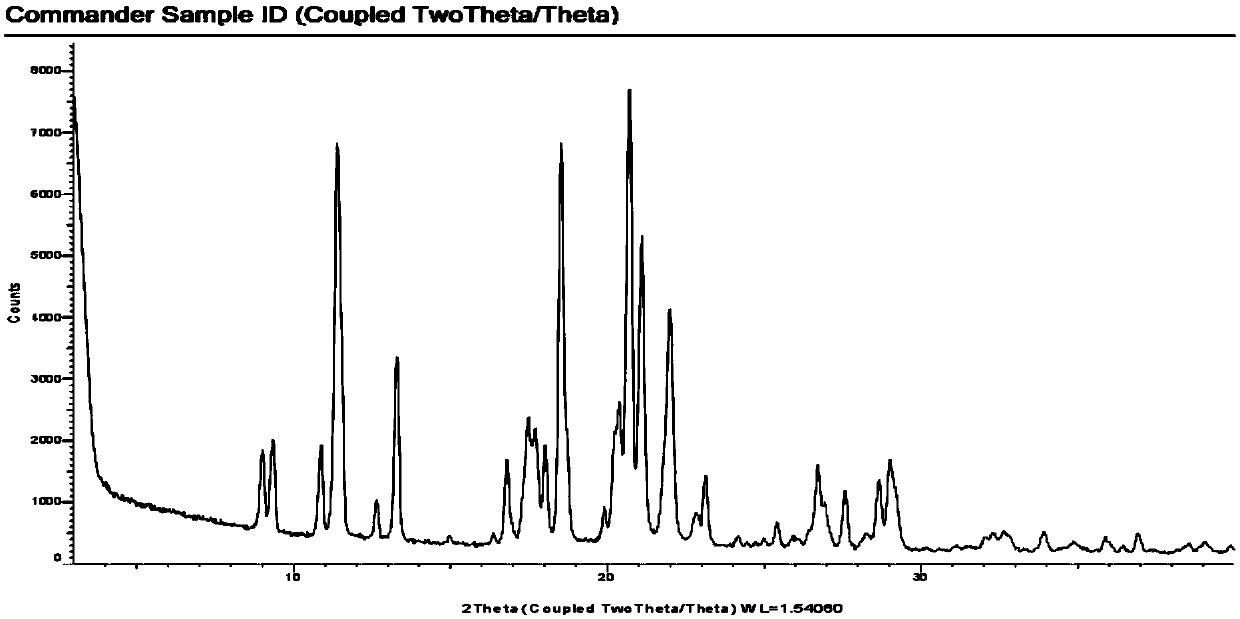
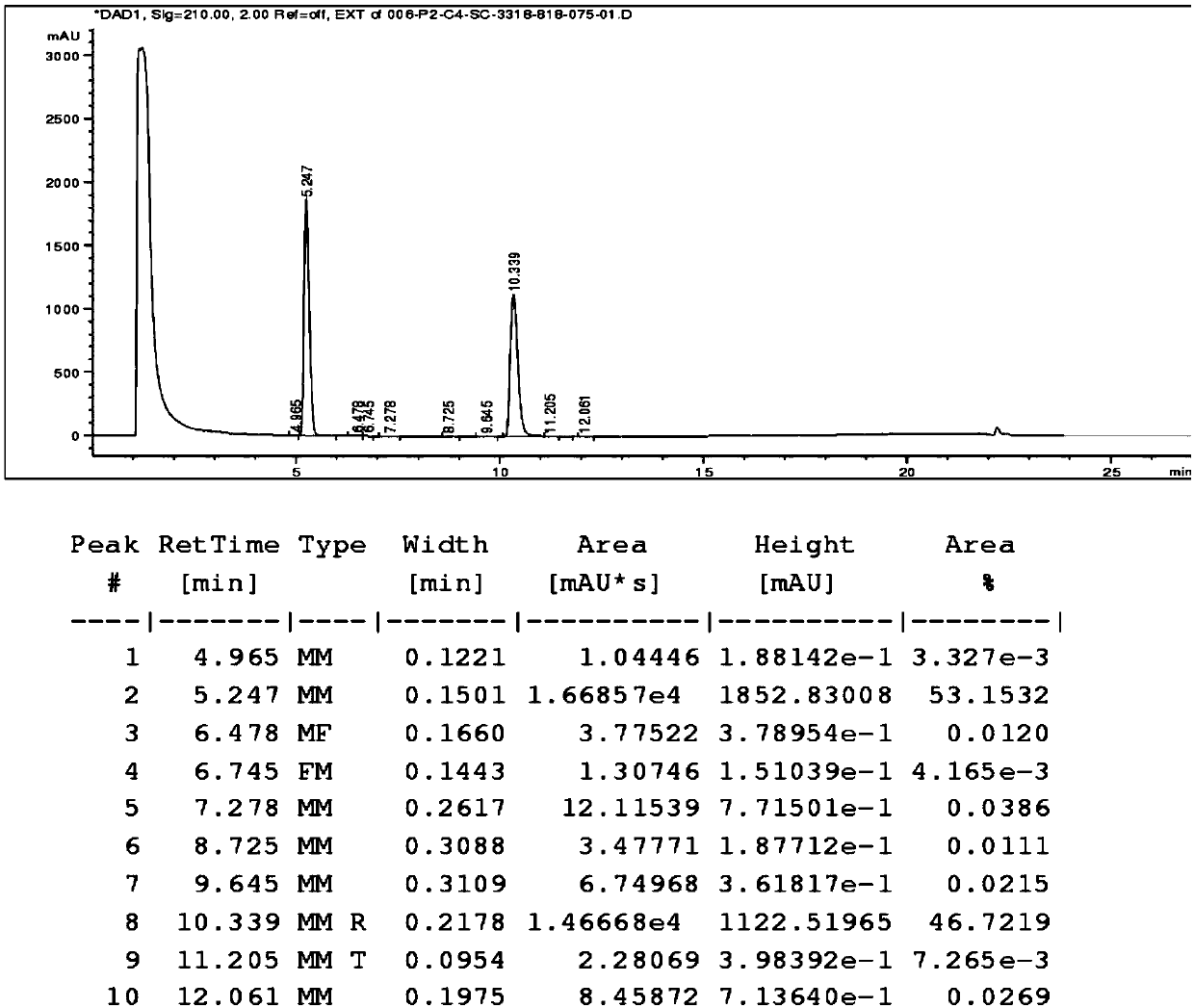
[0035] Weigh 500mg of escitalopram oxalate sample, add 25ml of water, and heat to 30°C to dissolve it completely; weigh 520mg of pamoic acid disodium salt, add 12.5ml of water, dissolve it completely at room temperature, and then Add 12.5ml of absolute ethanol and mix well. Add the water / ethanol mixed solution of pamoic acid disodium salt into the escitalopram oxalate aqueous solution dropwise at 30°C, solids appear immediately and the dispersion is good. After the dropwise addition, continue to stir for 2h and then filter. The filter cake was washed with 50ml of water, filtered with suction for 10min, and dried in vacuum. An 850 mg sample of escitalopram pamoate was obtained. Detected by HPLC, the purity is 99.66%
Embodiment 2
[0036] Implementation Example 2: Preparation of Donepezil Pamoate
[0037] Weigh 5g of escitalopram oxalate sample, add 40ml of water, and heat to 60°C to dissolve it completely; weigh 5.2g of pamoic acid disodium salt, add 25ml of water, dissolve it completely at room temperature, and then Add 25ml of absolute ethanol and mix well. Add the water / ethanol mixed solution of pamoic acid disodium salt into the escitalopram oxalate aqueous solution dropwise at 30°C, solids appear immediately and the dispersion is good. After the dropwise addition, continue to stir for 2h and then filter. The filter cake was washed with 50ml of water, filtered with suction for 10min, and dried in vacuum. An 8.5 g sample of escitalopram pamoate was obtained. As detected by HPLC, the purity was 99.71%.
Embodiment 3
[0038] Implementation Example 3: Preparation of Donepezil Pamoate
[0039] Weigh 50g of escitalopram oxalate sample, add 400ml of water, heat to 60°C to dissolve it completely; weigh 52g of pamoic acid disodium salt, add 250ml of water, dissolve it completely at room temperature, then add 250ml absolute ethanol, mix well. Add the water / ethanol mixed solution of pamoic acid disodium salt into the escitalopram oxalate aqueous solution dropwise at 30°C, solids appear immediately and the dispersion is good. After the dropwise addition, continue to stir for 2h and then filter. The filter cake was washed with 500ml of water, filtered with suction for 10min, and dried in vacuum. An 85 g sample of escitalopram pamoate was obtained. As detected by HPLC, the purity was 99.70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com