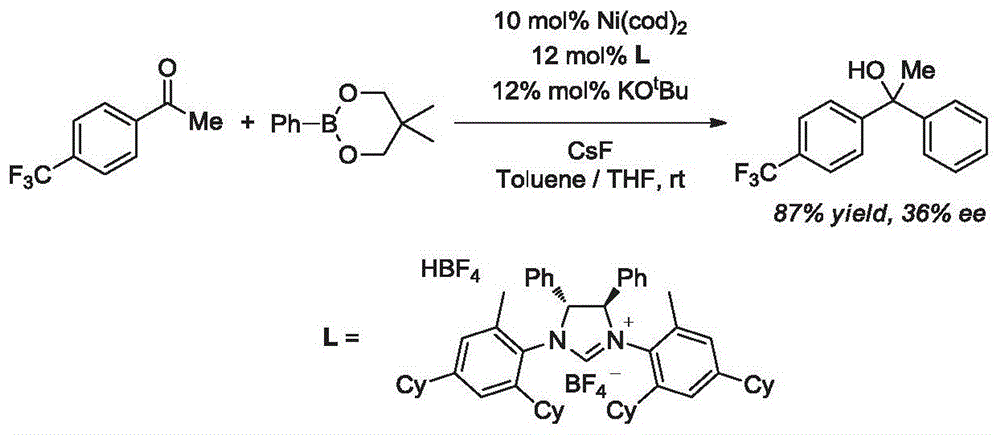
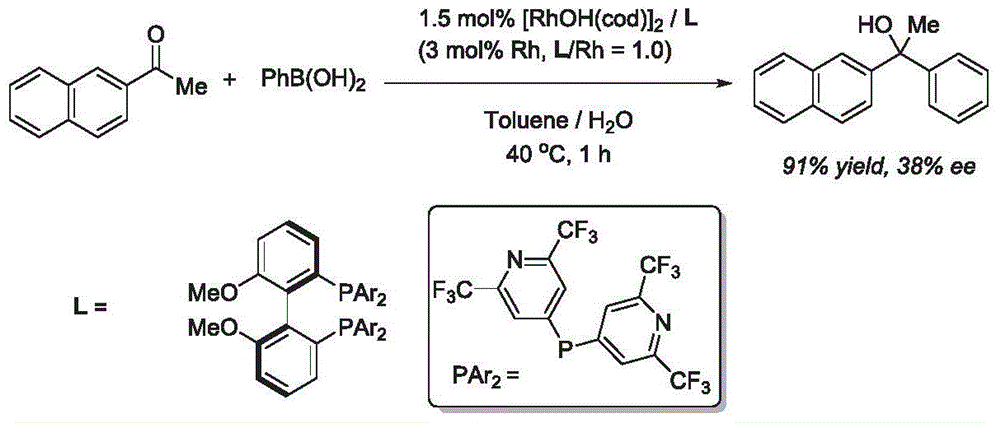
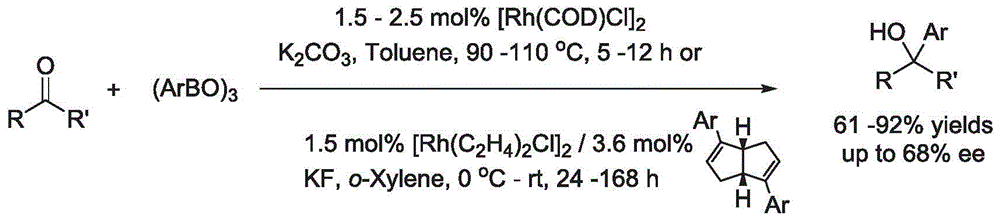
Synthesis method of aryl alcohol compound and Escitalopram
A synthetic method and compound technology, applied in the preparation of hydroxy compounds, amino hydroxyl compounds, organic chemical methods, etc., can solve the problems of high product yield and ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] With acetophenone 1a as substrate, 4-methoxyphenylboronic anhydride 2a as boron reagent, [Rh(C 2 h 4 ) 2 Cl] 2 Chiral diarylalkylmethanols were prepared as metal precursors under different conditions.
[0093]
[0094] Among them, the preparation methods of ligands L1-L4 refer to Angew.Chem.Int.Ed.2013, 52, 4235; Adv.Synth.Catal.2013, 355, 1297.
[0095] The reaction is as follows: Acetophenone (0.1mmol, 1equiv), arylboronic anhydride (0.2mmol, 2equiv), base (0.4mmol, 4equiv), additive (0.035mmol, 35mmol%), ligand (0.0036mmol, 3.6mol%) %) and [Rh(C 2 h 4 ) 2 Cl] 2(0.0015mmol, 1.5mol%) was mixed in a dry reaction tube, and after the nitrogen was replaced three times, 1.5mL of methyl tert-butyl ether was added under the protection of nitrogen, followed by reaction at 60°C or 100°C in an oil bath for 18h. After adding water (3 mL) to quench the reaction, it was extracted with ethyl acetate (10 mL×3). The organic phases were combined, washed with saturated brine...
Embodiment 2
[0100] With acetophenone 1a (12mg, 0.1mmol, 1equiv) as substrate, 4-methoxyphenylboronic anhydride 2a (80.4mg, 0.2mmol, 2equiv) as nucleophile, [Rh(C 2 h 4 ) 2 Cl] 2 (0.6mg, 0.0015mmol, 1.5mol%) as transition metal precursor, WingPhos (2.7mg, 0.0036mmol, 3.6mmol%) as ligand, cesium fluoride (45.6mg, 0.3mmol, 3equiv) as base, methyl Tert-butyl ether (1.5 mL) was used as a solvent, magnesium bromide (6.4 mg, 0.035 mmol, 0.35 equiv) was used as an additive, and the reaction was carried out at 100°C. The method for preparing chiral diarylalkylcarbinol described in the present invention is described in detail. The reaction was as follows: acetophenone 1a (12 mg, 0.1 mmol, 1 equiv), 4-methoxyphenylboronic anhydride 2a (80.4 mg, 0.2 mmol, 2 equiv), cesium fluoride (45.6 mg, 0.3 mmol, 3 equiv), bromine Magnesium chloride (6.4mg, 0.035mmol, 0.35equiv), WingPhos (2.7mg, 0.0036mmol, 3.6mmol%) and [Rh(C 2 h 4 ) 2 Cl] 2 (0.6mg, 0.0015mmol, 1.5mol%) were mixed in a dry reaction tube...
Embodiment 3
[0138] Synthesis of (S)-4-chloro-1-(2,4-dichlorophenyl)-1-(4-fluorobenzene)-1-butanol (5).
[0139]
[0140] 4-Chloro-1-(2,4-dichlorophenyl)-1-butanone (4) (50.3mg, 0.20mmol, 1equiv), 4-fluorophenylboronic anhydride (146.3mg, 0.40mmol, 2equiv) , Magnesium Bromide (12.9mg, 0.07mmol, 0.35equiv), Cesium Fluoride (121.5mg, 0.80mmol, 4equiv), (R,R,R,R)-WingPhos (5.4mg, 0.0072mmol, 3.6mol%) and [Rh(C 2 h 4 ) 2 C1] 2(1.2mg, 0.003mmol, 1.5mol%) was placed in a dry reaction tube, the nitrogen was purged three times, and freshly distilled methyl tert-butyl ether (1.5mL) was added. The reaction tube was placed in an oil bath at 80° C. for 10 hours. Then move to room temperature to cool, and add water to quench the reaction. Extracted with dichloromethane, combined organic phases, dried over anhydrous sodium sulfate, concentrated, and column chromatography gave the product (S)-4-chloro-1-(2,4-dichlorophenyl)-1-(4-fluorobenzene )-1-Butanol (5) (48.6 mg, 0.14 mmol, 70% yield, >99%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com