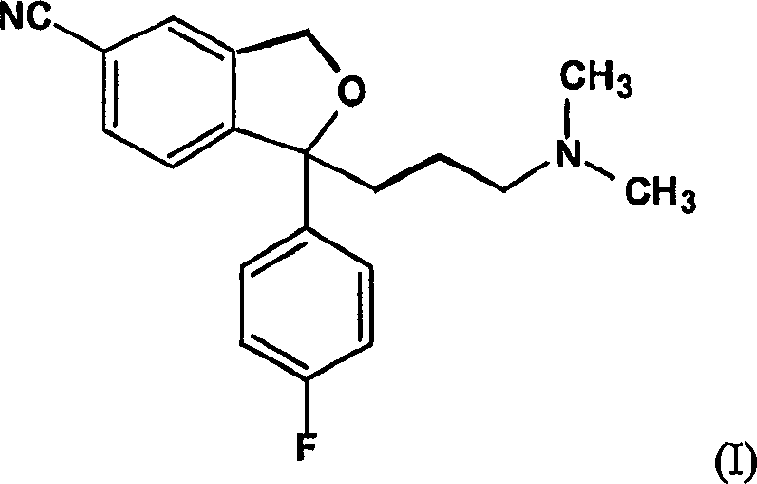
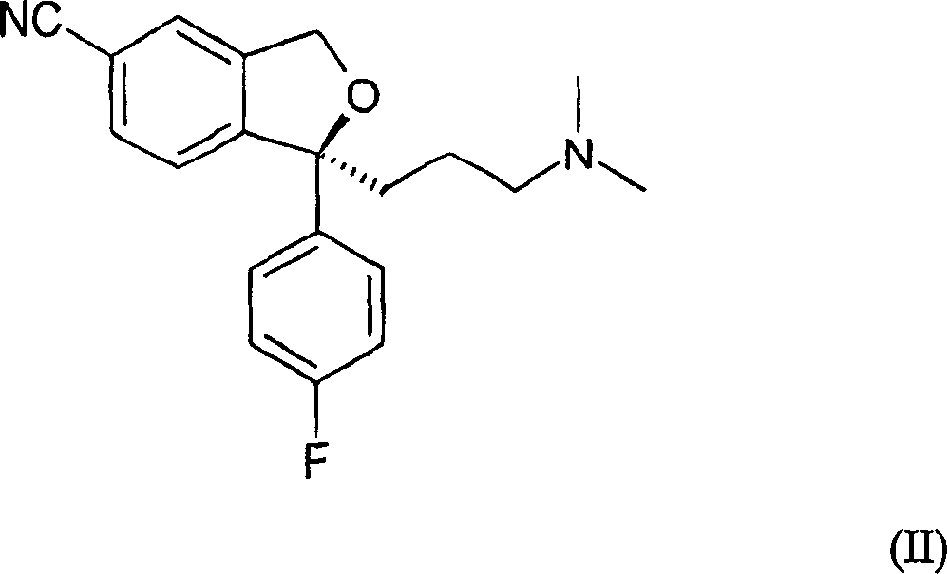
Crystalline escitalopram hydrobromide and methods for preparing the same
A technology of escitalopram and hydrobromide, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, digestive system, etc., and can solve problems such as failure to achieve success
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] A) Experiment with HCl gas: A 250 ml round bottom flask was charged with 5.7 g of escitalopram free base and 120 ml of isopropanol. The mixture was stirred until a homogeneous solution was obtained. The mixture was cooled to 5 °C and HCl gas was bubbled through for 20 minutes with cooling. The mixture is kept in the refrigerator overnight. No solid material formed. The mixture was then concentrated in vacuo to an oil, and the oily residue was dissolved in acetone (solution was 0.5 molar in acetone) by heating to 45°C. The flask was scraped to start nucleation and the solution became cloudy. The solution was cooled to 5°C overnight. The fluffy material was collected by filtration, transferred to a tan bottle and dried (50°C HI-VAC) to a powder. A sample of this powder is exposed to air, and when it absorbs moisture from the air, it turns into an oil. Recrystallization of the oil was attempted using various solvents. However, none of these trials resulted in a soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com