Method for synthesizing clofarabine
A synthesis method and technology of clofarabine are applied in the directions of drug combination, sugar derivatives, organic chemistry, etc., which can solve the problems of unsuitable industrial production, high production cost, low total yield and the like, and achieve improved yield and less reaction impurities. , the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
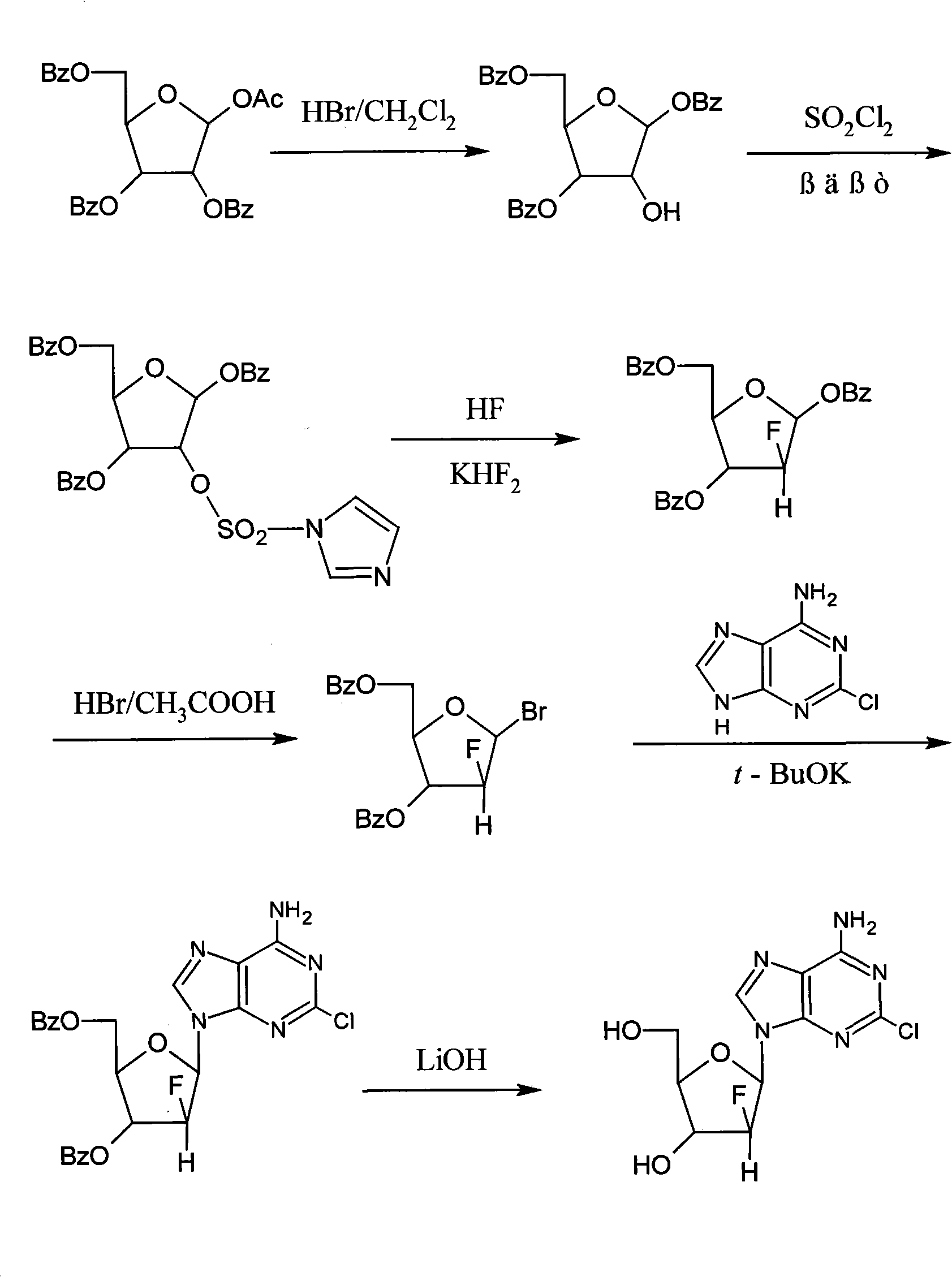
[0022] (1) Preparation of 2-deoxy-1,3,5-tri-oxo-benzoyl-β-D-ribofuranose
[0023] Under a nitrogen atmosphere, 1.6 Kg of 1-acetyl-2,3,5-tri-oxo-benzoyl-β-D-ribofuranose and 10 L of dichloromethane were added into a 20 L reactor, and stirred and mixed evenly. The temperature was lowered to -10~-5°C, and 8L of 0.4Mol / L hydrogen bromide in dichloromethane solution was added dropwise. After the dropwise addition was completed, the insulation reaction was continued for 5 h. Washed twice with water, separated, and the organic layer was spin-dried to obtain 1.2 Kg of a white solid with a yield of 81%.
[0024] (2) Preparation of 2-deoxy-2-imidazolesulfonyl-1,3,5-tri-oxo-benzoyl-β-D-ribofuranose
[0025] Under nitrogen atmosphere, add 1.2Kg of 2-deoxy-1,3,5-tri-oxo-benzoyl-β-D-ribofuranose, 18L of dichloromethane and 3L of N,N-dimethylformamide to 30L Reactor, stirring to dissolve. The temperature was lowered to -15~-10°C, and 900 g of sulfuryl chloride was added dropwise. After ...
Embodiment 2
[0035] (1) Preparation of 2-deoxy-1,3,5-tri-oxo-benzoyl-β-D-ribofuranose
[0036] Under a nitrogen atmosphere, 0.8Kg of 1-acetyl-2,3,5-tri-oxo-benzoyl-β-D-ribofuranose and 5L of dichloromethane were added into a 10L reaction kettle, and stirred and mixed evenly. The temperature was lowered to 5-15°C, and 4L of 0.4Mol / L hydrogen bromide in dichloromethane solution was added dropwise. After the dropwise addition was completed, the insulation reaction was continued for 10 h. Washed twice with water, separated, and the organic layer was spin-dried to obtain 0.6 Kg of a white solid with a yield of 81%.
[0037] (2) Preparation of 2-deoxy-2-imidazolesulfonyl-1,3,5-tri-oxo-benzoyl-β-D-ribofuranose
[0038] Under nitrogen atmosphere, add 0.6Kg of 2-deoxy-1,3,5-tri-oxo-benzoyl-β-D-ribofuranose, 9L of dichloromethane and 1.5L of N,N-dimethylformamide to 20L Reactor, stirring to dissolve. The temperature was lowered to -40~-30°C, and 450 g of sulfuryl chloride was added dropwise. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com