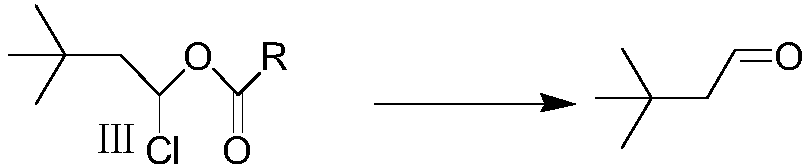Patents
Literature
33results about How to "Less impurities in the reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of N(2)-L-alanyl-L-glutamine
ActiveCN103626839APrice stabilityMarket sales trend ups and downsPeptide preparation methodsL-alanyl-l-glutamineChloride
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of N(2)-L-alanyl-L-glutamine. The preparation method of the N(2)-L-alanyl-L-glutamine comprises the following steps of (1) preparing L-phthaloyl-alanyl chloride; (2) preparing phthaloyl-L-alanyl-L-glutamic acid; (3) preparing phthaloyl-L-alanyl-L-glutamic acid anhydride; (4) preparing phthaloyl-L-alanyl-L-glutamine; (5) preparing an N(2)-L-alanyl-L-glutamine crude product; (6) preparing an N(2)-L-alanyl-L-glutamine refined product. The product obtained through the final deprotection process of the preparation method as a pilot plant test or a production scale process route is higher in purity; the liquid-phase purity of the product is higher than 99.9% through primary purification, and the product is low in impurity content.
Owner:JINAN CHENGHUI SHUANGDA CHEM
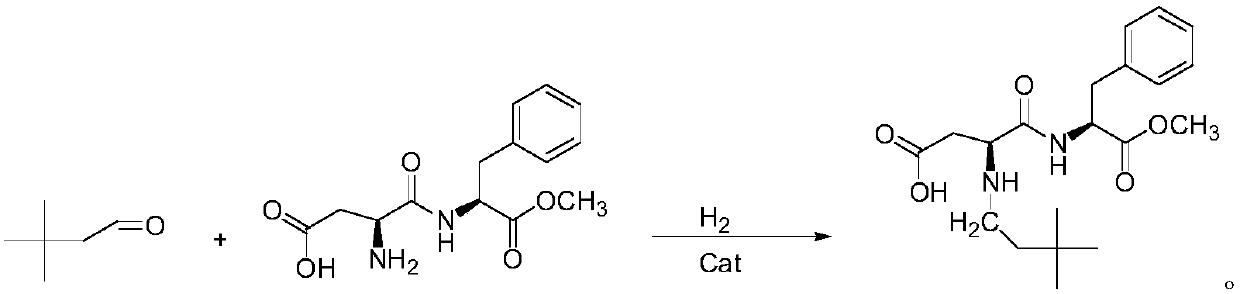
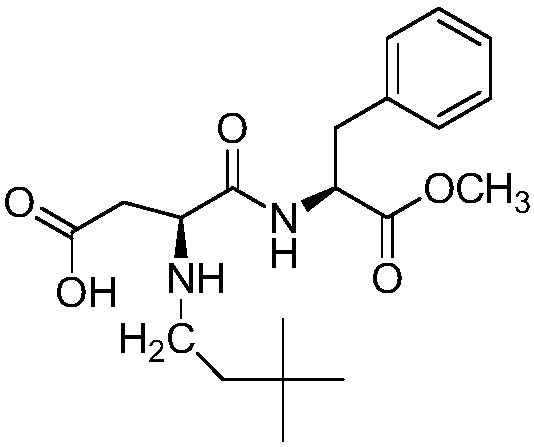
Synthesis method of neotame
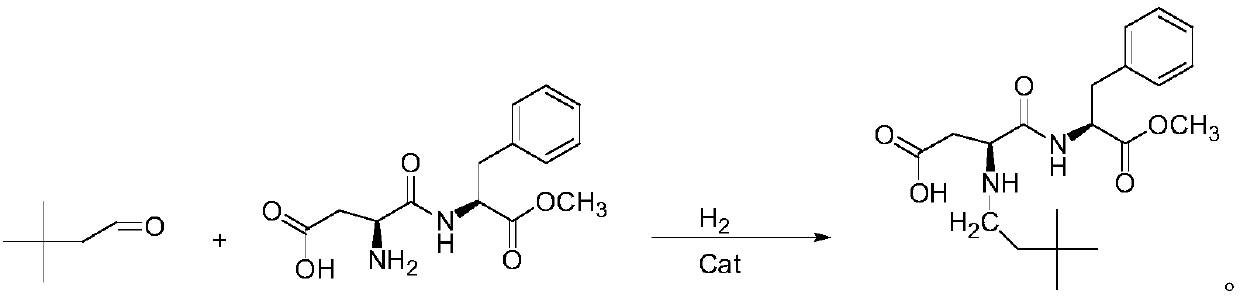
The invention relates to a synthesis method of neotame. The method comprises the following steps: (1) adding aspartame and 3,3-dimethyl-butyl aldehyde to an organic solvent in a vacuum state, reacting at the reaction temperature of 20-50 DEG C for 5-24 hours, carrying out concentration and separation at 0-50 DEG C after reaction is ended, concentrating to obtain white paste, adding deionized water to devitrify, thus obtaining a neotame intermediate imine in a centrifuging manner; (2) adding imine and an organic solvent to a hydrogenation reactor, introducing hydrogen until the pressure is 0.1-0.5MPa, adding a catalyst, reacting at the temperature of 35-40 DEG C for 5-30 hours, filtering and removing the catalyst, concentrating the filtrate and removing an organic solvent, and recrystallizing and purifying by water, methanol or ethanol, so as to obtain the neotame. According to the synthesis method, the defects in the prior art are overcome, high-purity imine is generated, the activity of the catalyst is reduced, the selectivity of the catalyst on the imine is increased, the service life of the catalyst is prolonged, the reaction impurity is reduced, and the product purity is improved.
Owner:SHANDONG BENYUE BIOTECH
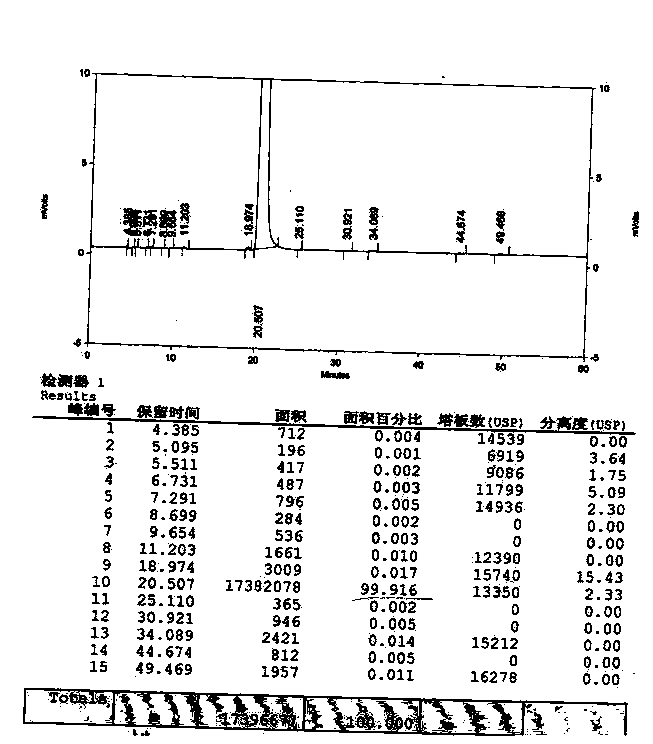
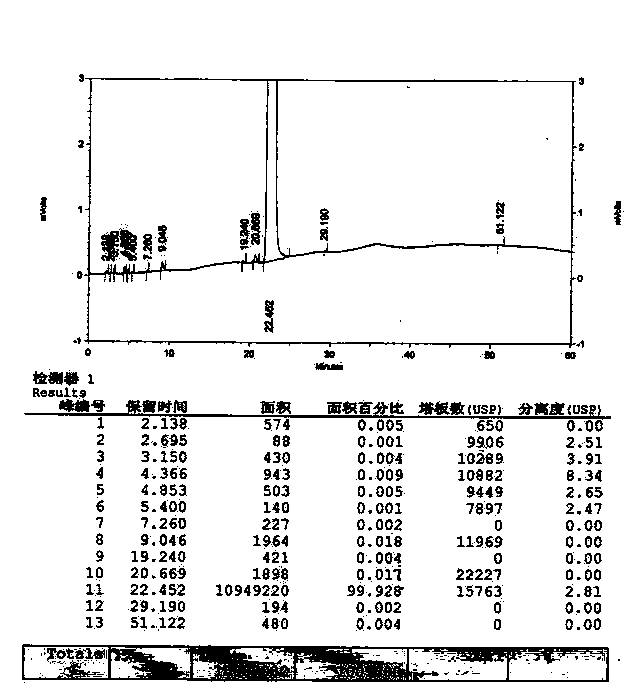
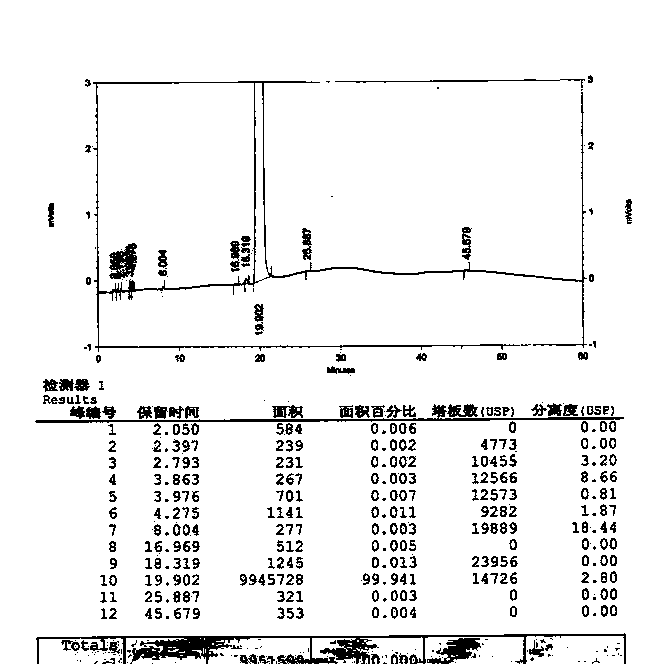
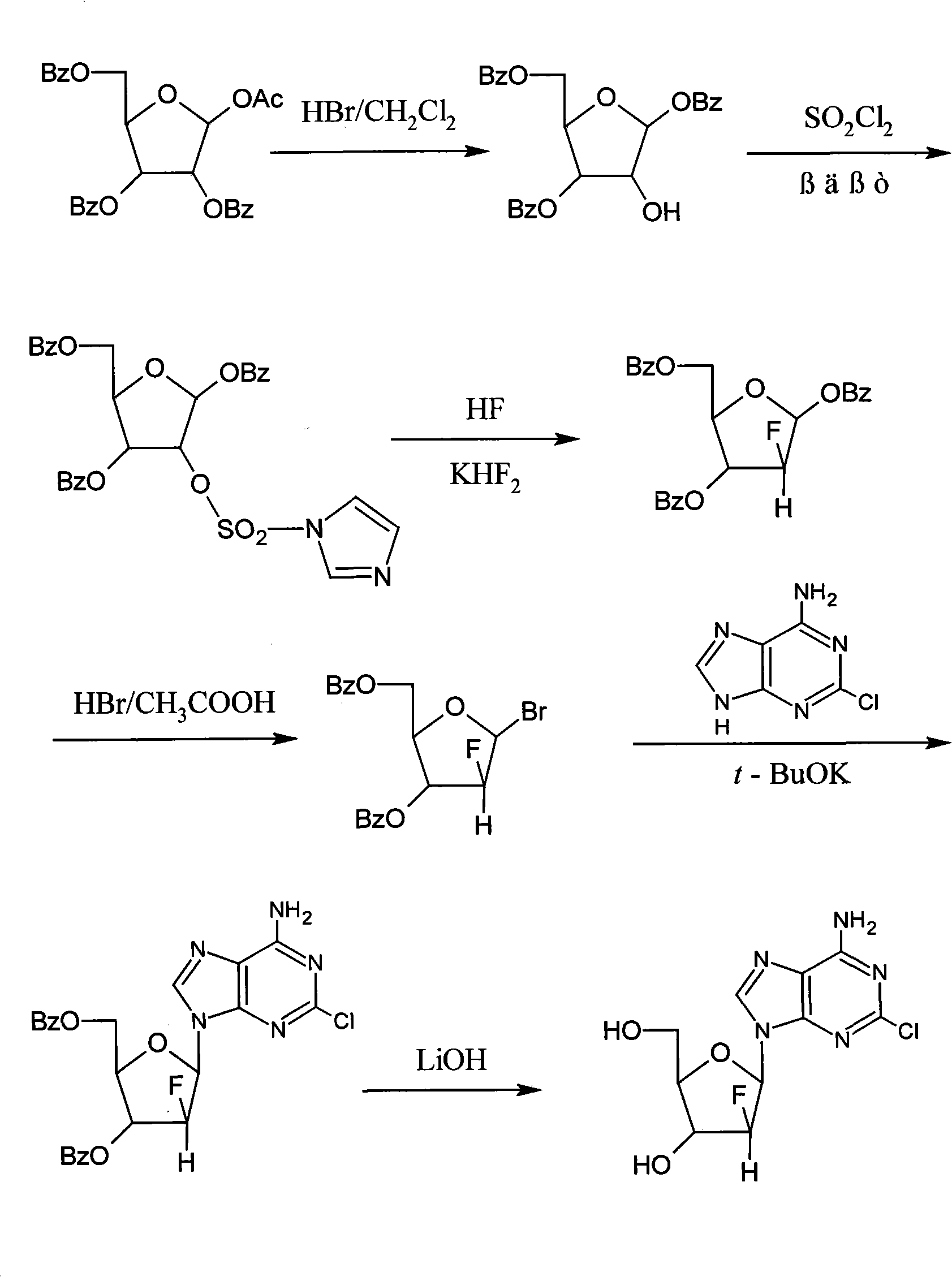
Method for synthesizing clofarabine
InactiveCN101265284AHigh selectivityHigh yieldSugar derivativesAntineoplastic agentsHydrobromidePhenacyl
The invention provides a preparation method of clofarabine, which includes allowing 1-acetyl-2,3,5-tri-o-benzoyl-Beta-D-ribofuranose as the initial raw material and dichloromethane solution of hydrobromide to perform the rearrangement reaction, reacting with sulfuryl chloride and imidazole, performing the fluoridation reaction in the presence of hydrogen fluoride aqueous solution and potassium hydrogen fluoride, and performing bromination reaction in acetic acid solution of hydrogen bromide, condensing with 2-chloro adenosine in alkaline condition, and removing benzoyl in the presence of lithium hydroxide to obtain clofarabine. Compared with prior art, the method has the advantages of high yield of each step, higher total yield, and easily realized industrialized production.
Owner:深圳万乐药业有限公司
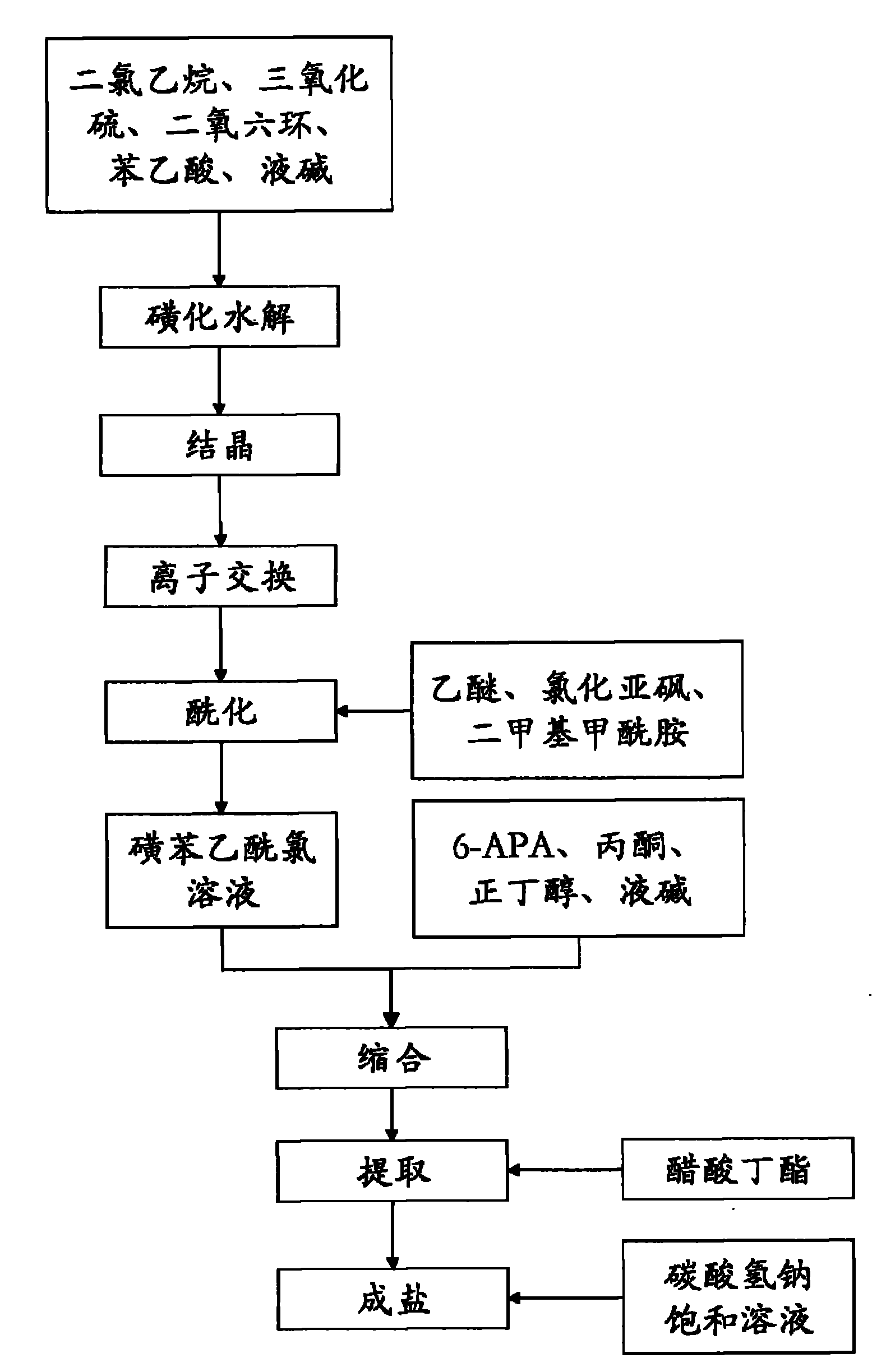
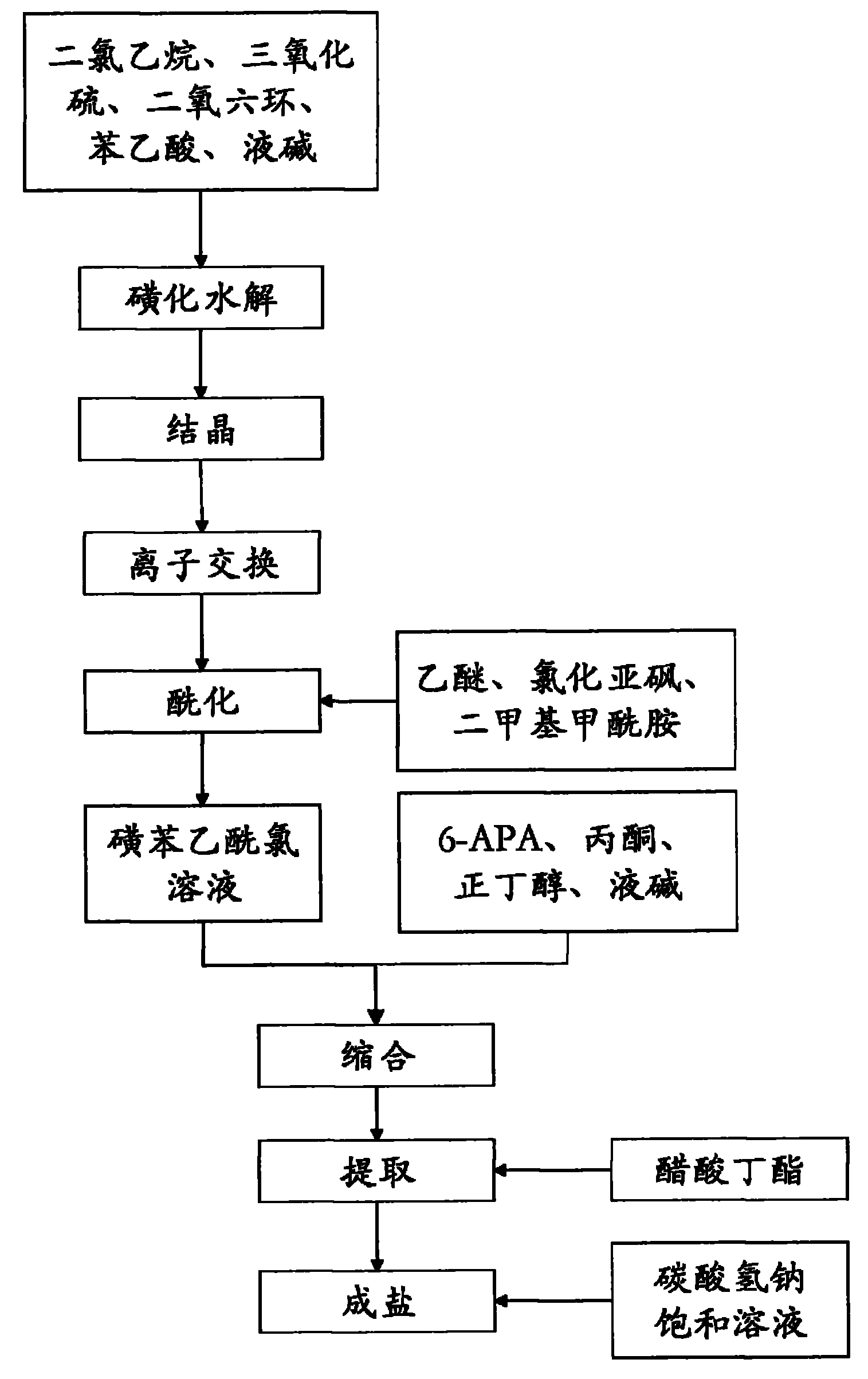
Preparation methods of sulbenicillin sodium and injection thereof
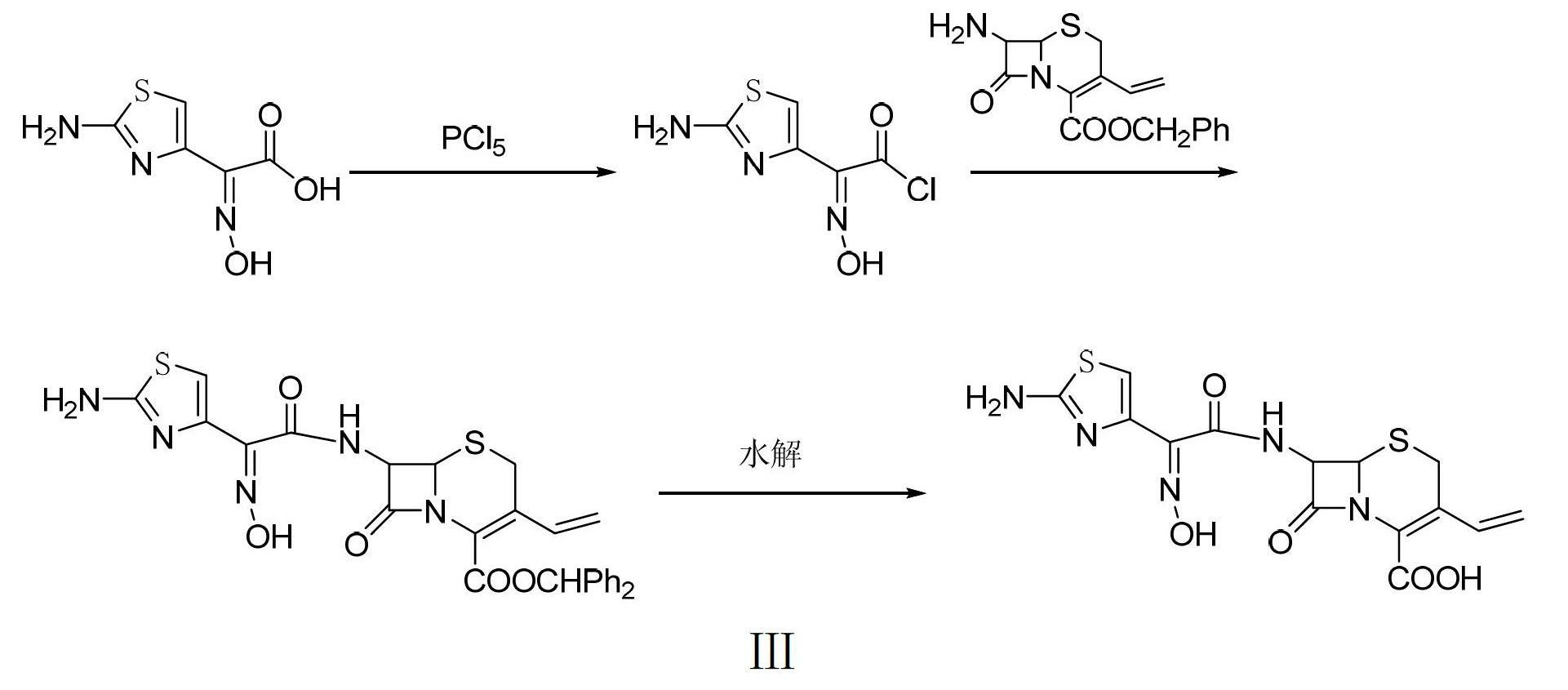
InactiveCN101805356ASimple stepsLess impurities in the reactionAntibacterial agentsOrganic chemistryIonIon exchange
The invention discloses a preparation method of sulbenicillin sodium, which comprises the following steps of: sulfonating, hydrolyzing, crystallizing, ion-exchanging, acidylating, condensing, extracting and salifying to obtain sulbenicillin sodium. The method has the advantages of simple technological steps, fewer reaction impurities and high product purity, and the yield is higher than 50%. Meanwhile, the invention also relates to a preparation method of a sulbenicillin sodium injection.
Owner:HUNAN ERKANG XIANGYAO PHARMA
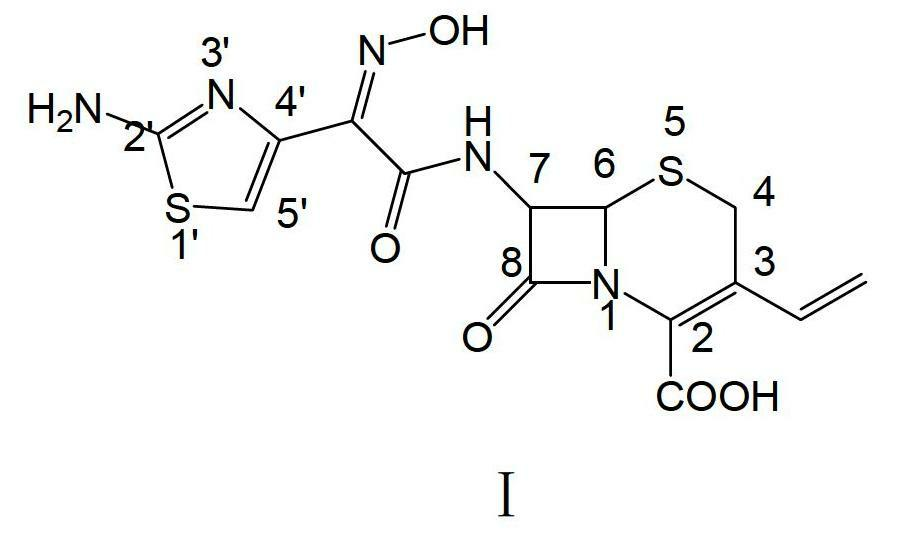
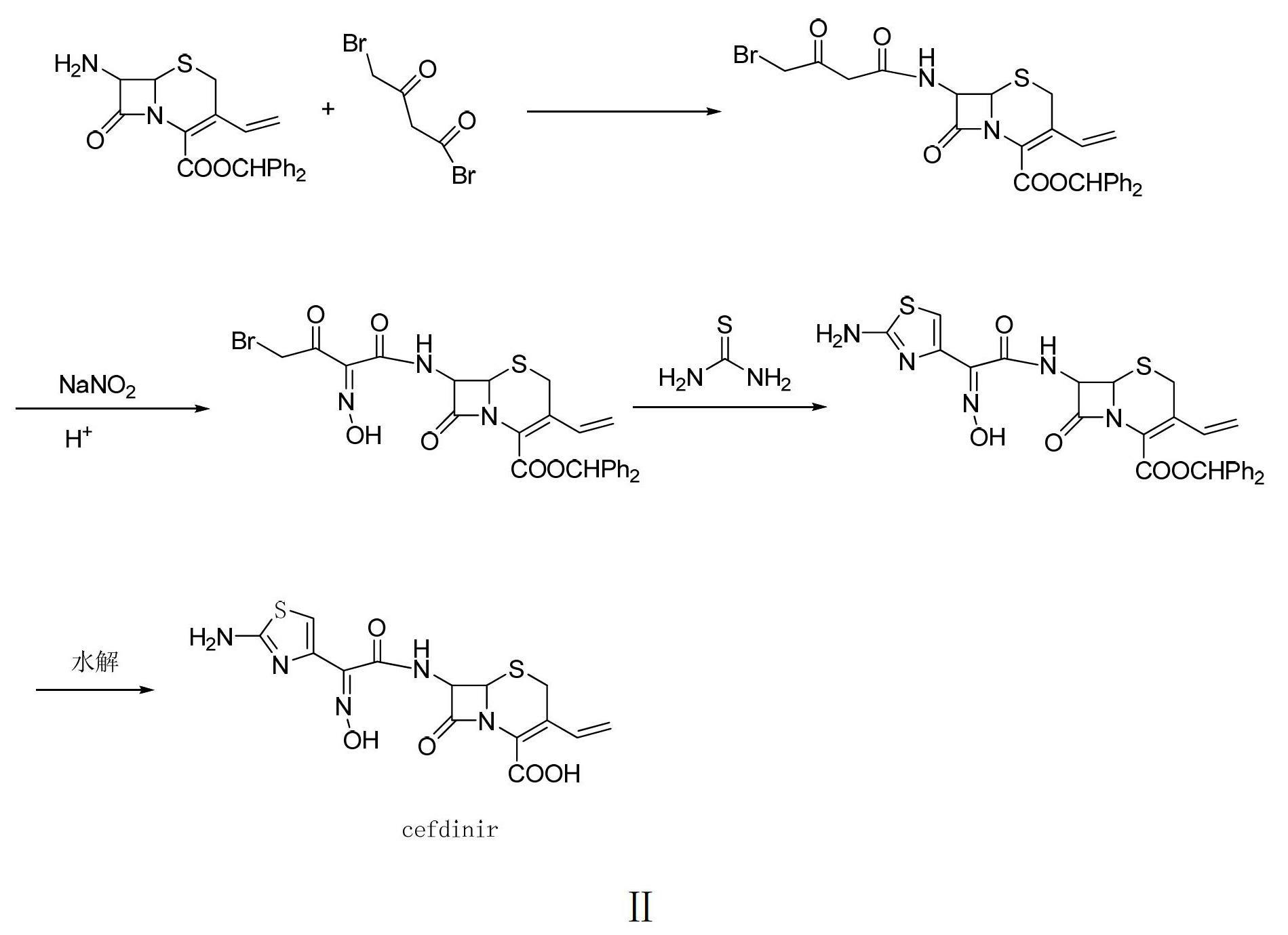
Preparation method of cefdinir
ActiveCN102659817AIncrease the speed of the condensation reactionShort reaction timeOrganic chemistryOrganic solventWater soluble
The invention relates to a preparation method of cefdinir, which comprises the following steps: dissolving T15-AE active ester and 7-AVCA in a water system containing water-soluble organic solvent, carrying out condensation reaction at normal temperature under the action of organic alkali, and directly extracting without regulating the pH value to obtain a cefdinir intermediate; under the combined actions of mixed alkalis, efficiently removing an ester group protective agent from the cefdinir intermediate, and precipitating cefdinir compound salts; and dissolving the obtained cefdinir compound salts in water for decolorization, adding the water-soluble organic solvent, and acidifying to crystallize, thereby obtaining the cefdinir product. The invention is simple to operate, has the advantages of high product purity, low production cost and low environmental pollution, and is suitable for large-scale production.
Owner:ZHEJIANG APELOA TOSPO PHARMA +1
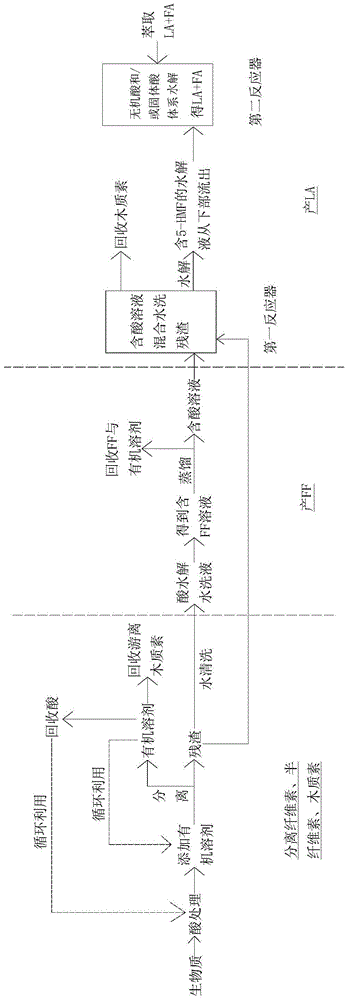
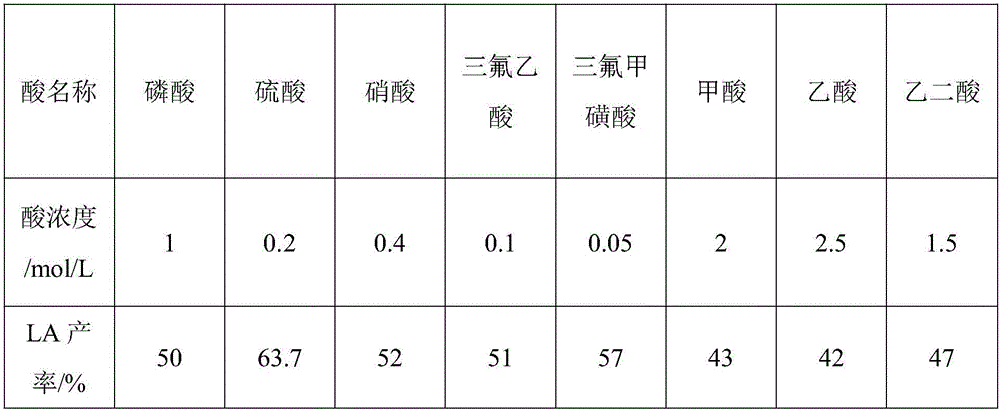
Method for grading biomass, preparing furfural and preparing levulinic acid through two steps
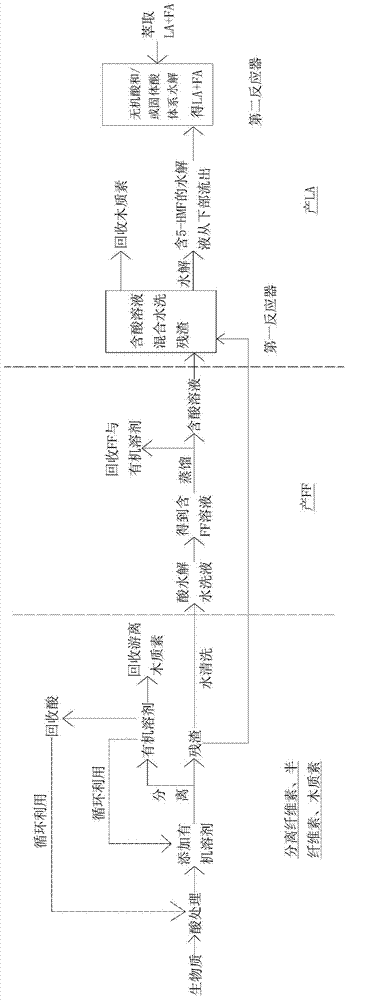
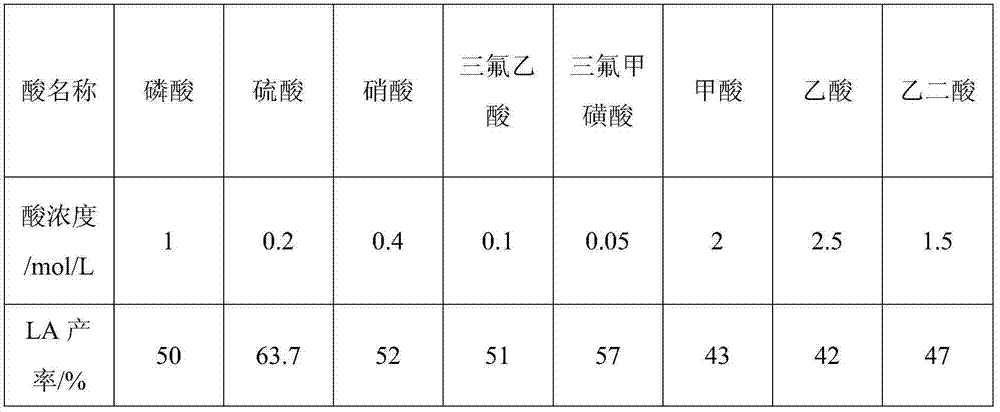
ActiveCN104292193AImprove extraction efficiencyReduce generationOrganic compound preparationChemical recyclingChemical structureFurfural
The invention provides a method for grading biomass, preparing furfural and preparing levulinic acid (LA) through two steps. The method comprises the following steps: processing a biomass raw material by using an acid / organic solvent combination system to separate lignose, hemicellulose and cellulose, converting hemicellulose into xylooligosaccharide, and converting cellulose into amorphous cellulose; extracting xylooligosaccharide by water, and converting xylooligosaccharide into furfural; and carrying out two-step acid hydrolysis on amorphous cellulose-based residues to make amorphous cellulose in residues converted into 5-HMF (5-hydroxymethylfurfural), introducing the 5-HMF to a second reactor with acidic environment, and carrying out acid hydrolysis to generate the LA. The method has the advantages of extremely high recovery rate / yield of sugar, FF and LA, few side reactions, high recovery rate and small chemical structure change of lignin, less water consumption, fast reaction, low requirements on a reactor, recycling of all reagents, and environmental protection.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
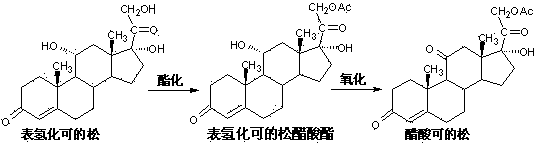
Preparation method of cortisone acetate
The invention discloses a preparation method of cortisone acetate. According to the method, hydrocortisone is taken as an initiator, and preparation is performed through steps including acetylation, oxidization and the like. Sodium hypochlorite which is low in cost and environment-friendly is used for replacing chromic anhydride which is high in cost and larger in toxicity and serves as an oxidizing agent in an oxidation reaction, and a catalyst which cannot be recovered and recycled is not required in an oxidation reaction process. The preparation method of the cortisone acetate has the advantages that the production cost is reduced, the production operation is simplified, environmentally hazardous chromium ion wastewater is not discharged, and the method is suitable for industrial production.
Owner:HUAZHONG PHARMA
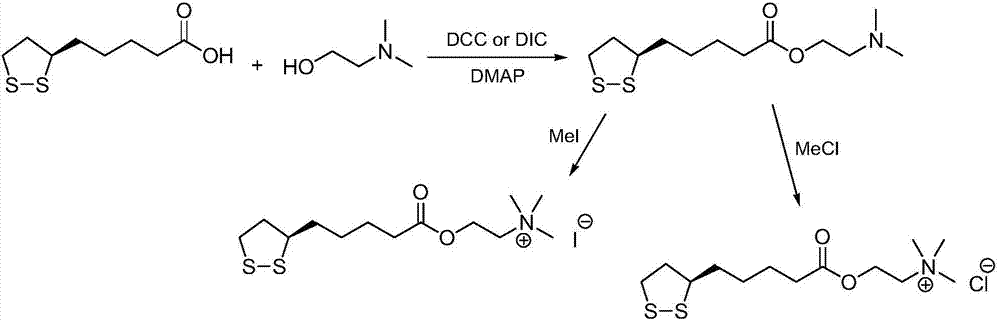
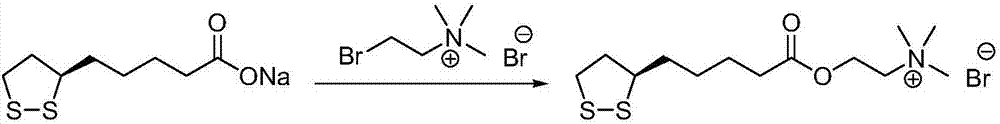
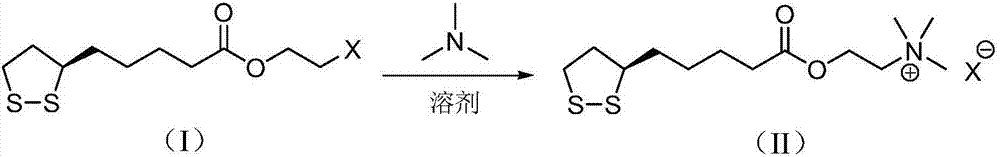
Preparation method of R-lipoic acid cholinesterase halide
InactiveCN107089967ALess impurities in the reactionEasy to operateOrganic chemistry methodsChemical synthesisSolvent
The invention provides a preparation method of an R-lipoic acid cholinesterase halide and belongs to the technical field of medicinal chemical synthesis. R-lipoic acid-2-halogen ethyl ester I and trimethylamine react in a solvent to generate quaternary ammonium salt, so that an R-lipoic acid cholinesterase halide II is obtained. Impurities during reaction are fewer, and operations such as aftertreatment and purification are simplified; raw materials and the used reagent are easy to get and safe to use, and a reaction route is reasonable, so that the preparation method is applicable to industrial enlarged production; and no pollutant is produced in a preparation process, so that the green environmental protection effect is realized.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
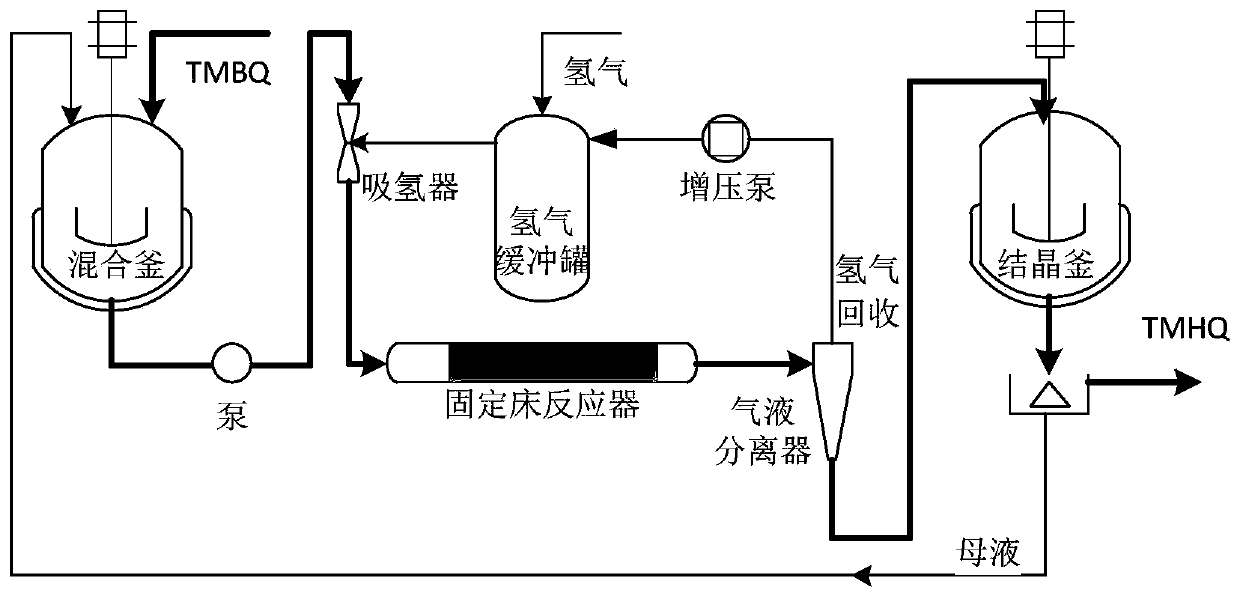
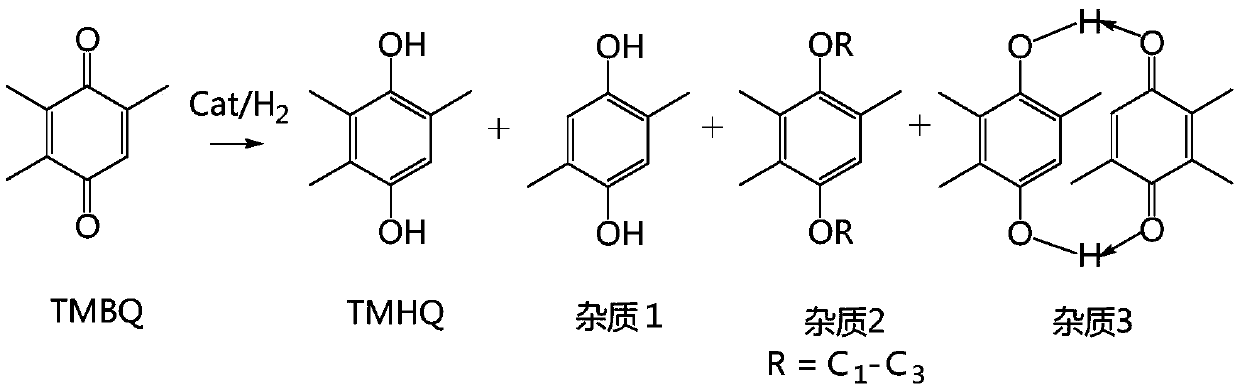
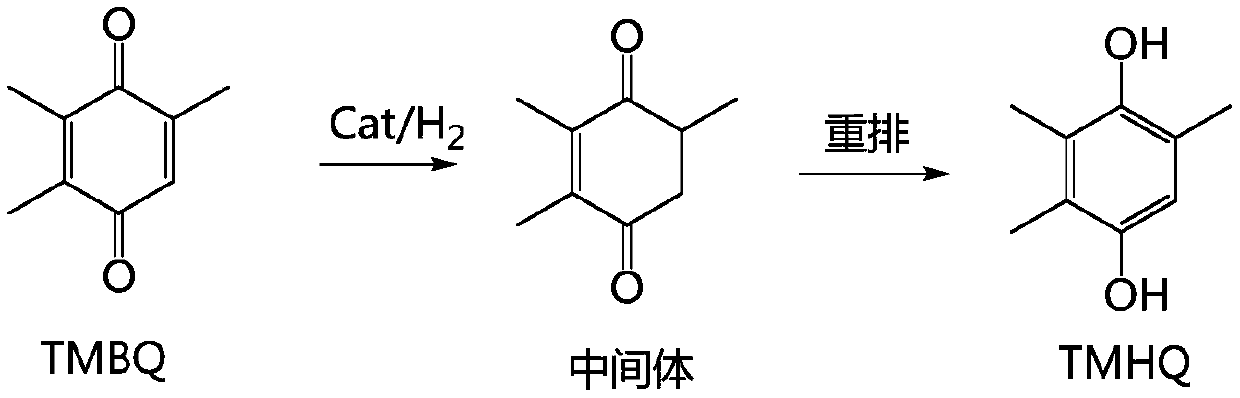
Synthesis method and device of 2,3,5-trimethylhydroquinone
InactiveCN111253218AHigh amount of hydrogenControl contentLiquid degasificationOrganic chemistryAlkanePtru catalyst
The invention discloses a synthesis method and device of 2,3,5-trimethylhydroquinone. According to the synthesis method for 2,3,5-trimethylhydroquinone, 2,3,5-trimethylbenzoquinone (TMBQ) and an alcohol-aromatic hydrocarbon or alcohol-alkane mixed solvent system are mixed, then a reaction solution is fully mixed with hydrogen through a hydrogen absorber, then the mixture enters a fixed bed filledwith a noble metal catalyst to complete a hydrogenation reaction, and 2,3,5-trimethylhydroquinone (TMHQ) is obtained. According to the technical scheme provided by the invention, the reaction selectivity is improved, the side reaction is effectively inhibited, the impurity content of the product is reduced, the purity of 2,3,5-trimethylhydroquinone (TMHQ) is improved, the production process is simplified, the emission of three wastes is reduced, and the method has good environmental protection benefits.
Owner:SHANGYU NHU BIOCHEM IND +2
Method for efficiently synthesizing Neotame
The invention provides a method for efficiently synthesizing Neotame. Aspartame, 3,3-dimethylbutyraldehyde and organic solvent are synthesized into the Neotame under the hydrogenation of catalyst. Themethod has the advantages of low raw material cost, short reaction time, simpleness in operation and few reaction impurities, the yield of a finished product is 95% or more, the quality of the finished product conforms to the requirement of the united states pharmacopeia, and the finished product is favorable for industrial production and has a good production prospect.
Owner:NANTONG CHANGHAI FOOD ADDITIVE
Preparation method of 5'-adenylic acid
ActiveCN107602648AImprove conversion rateFair priceSugar derivativesSugar derivatives preparationAdenosineSynthesis methods
The invention discloses a preparation method of 5'-adenylic acid. The preparation method of 5'-adenylic acid comprises the following steps: performing synthesis reaction on powdered adenosine and polyphosphoric acid which serve as raw materials under the condition of no solvent, adding pure water after the synthesis reaction to perform hydrolysis, and refining the crude product obtained by the hydrolysis, wherein the mass ratio of the powdered adenosine to the polyphosphoric acid to the pure water is 1:(2-4):(2-4). The preparation method of 5'-adenylic acid has the following advantages: the solvent-free synthesis method is adopted, the reaction impurities are few, the condition is mild and pollution is avoided; the product yield is 90 percent or above, the product content is more than 99 percent and the conversion rate of the raw materials is high; and the operation steps are simple, the raw materials are wide in source and the price is moderate, so the preparation method of 5'-adenylic acid can be popularized and implemented on a large scale.
Owner:江苏香地化学有限公司
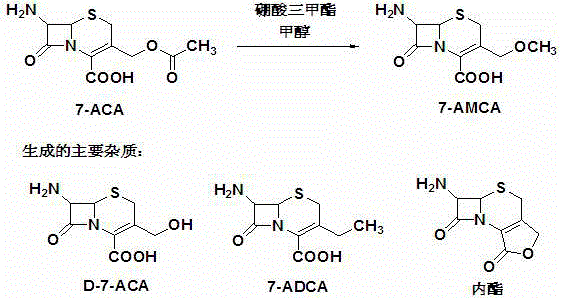
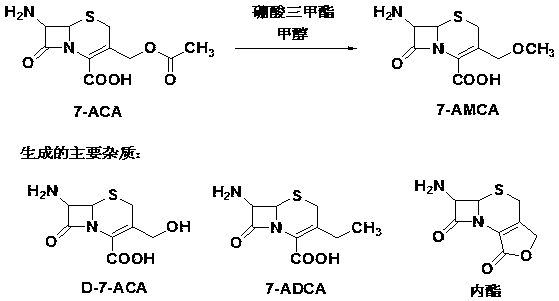
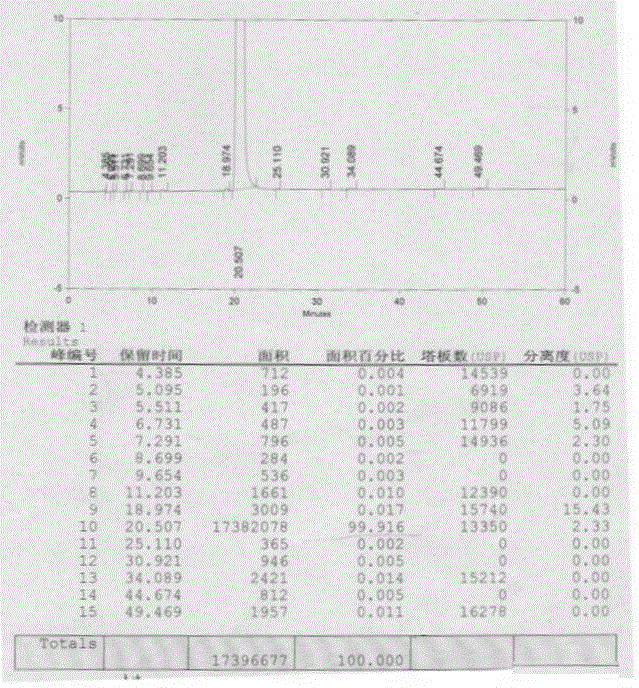
Preparation method of 7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid
The invention discloses a preparation method of 7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid, and relates to the technical field of preparation of pharmaceutical intermediates. The method includes the steps that under the protection of nitrogen, 7-aminocephalosporanic acid, a silane reagent and an imidazole catalyst are stirred for 0.5-2 h at 30-35 DEG C in a water-soluble organic solvent; then, the mixture is cooled to 0-5 DEG C, methanesulfonic acid, trimethyl borate and methyl alcohol are added, a methoxylation reaction is conducted with the temperature controlled to be 0-5 DEG C, and 7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid is obtained, wherein the silane reagent is hexamethyldisilazane or trimethylchlorosilane or N,O-bis(trimethylsilyl)acetamide or dimethoxydimethylsilane. According to the method, the product purity is high, the impurity content is low, operation is easy and convenient, refining steps are simplified, production cost is reduced, and the method is suitable for industrial production.
Owner:湖北凌晟药业股份有限公司
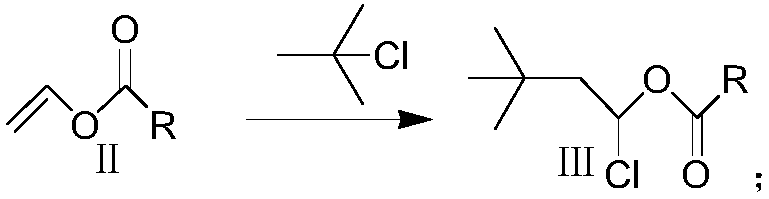
Method for synthesizing 3,3-dimethyl butyraldehyde
PendingCN108794312ALow costHigh yieldOrganic compound preparationCarboxylic acid esters preparationOrganic solventSolid acid
The invention provides a technological method for synthesizing 3,3-dimethyl butyraldehyde. The technological method comprises the following steps: adding an organic solvent and a solid acid catalyst into a container under the protection of nitrogen gas; dropwise adding tert-butyl chloride; then dropwise adding alkyl acid type vinyl ester; carrying out heat-insulation reaction, then filtering to remove a catalyst; then decompressing and distilling an organic layer; then adding alkali or acid, and heating, refluxing and hydrolyzing to obtain a crude product; then rectifying the crude product toobtain a 3,3-dimethyl butyraldehyde finished product. The technological method has the advantages of low raw material cost, mild reaction conditions, simplicity in operation and few side reaction, safety and environmental protection, and industrialized production is facilitated.
Owner:NANTONG CHANGHAI FOOD ADDITIVE
Method for preparing 8-quinoline carboxylic acid and derivatives of 8-quinoline carboxylic acid
ActiveCN112174887AOxidation process conditions are mildImprove product qualityOrganic chemistryChemical recyclingPtru catalystPhysical chemistry
The invention discloses a method for preparing 8-quinoline carboxylic acid and derivatives thereof, which comprises the following steps: by using a compound I as a raw material, reacting with an oxidant at the temperature of 50-150 DEG C under normal pressure in a solvent under the action of a Cu-Co-X ternary composite catalyst to obtain a compound II; according to the method, the efficient Cu-Co-X ternary composite catalyst is adopted, the reaction is carried out at the temperature of 50-150 DEG C under normal pressure, the reaction condition is mild, the oxidation yield is remarkably increased, near-zero emission of three wastes is realized, the pollution problem is effectively solved, and the production cost is greatly reduced.
Owner:JIANGSU KUAIDA AGROCHEM
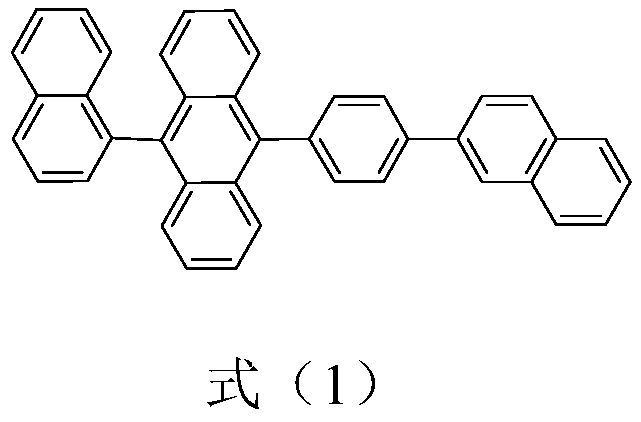
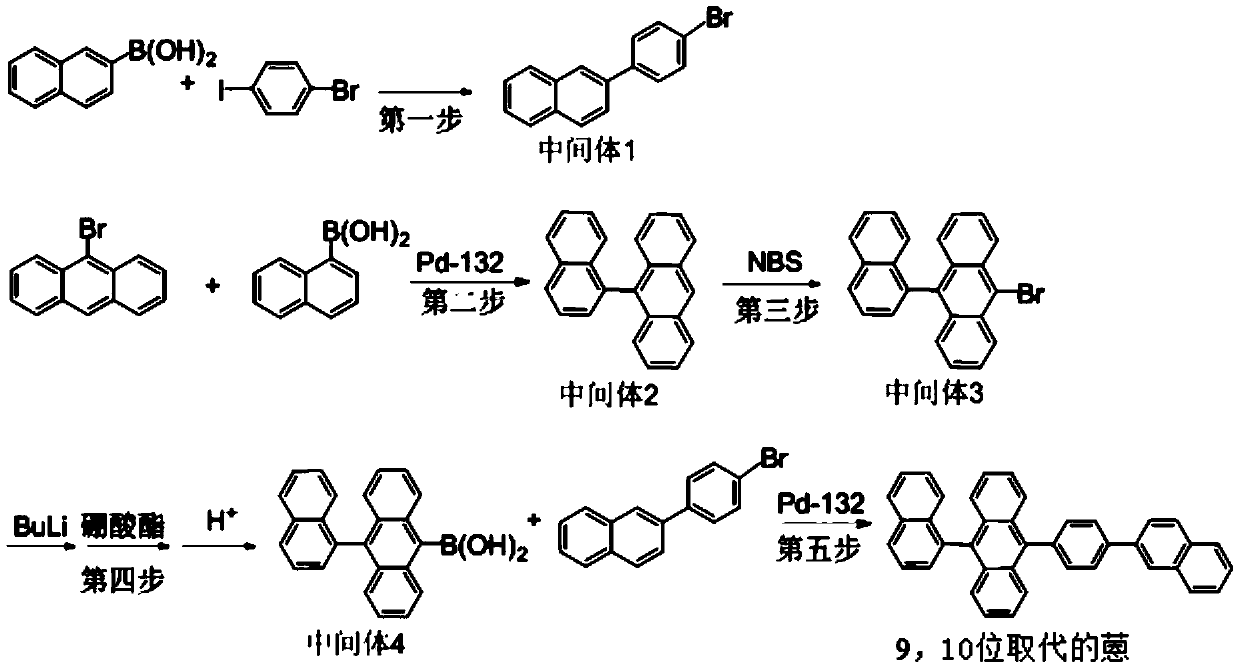
Preparation method and purification method of 9,10-substituted anthracene
InactiveCN110963876AHigh catalytic activityImprove conversion rateGroup 3/13 element organic compoundsHydrocarbonsAnthracenePtru catalyst
The invention belongs to the technical field of organic synthesis and catalysis, and particularly relates to a preparation method for synthesizing 9-(naphthalene-1-yl)-10-(4-(naphthalene-2-yl)phenyl)anthracene through a five-step reaction, and a purification method. The method provided by the invention has the advantages of less catalyst dosage, high synthesis yield, less reaction by-products (impurities) (the content of removed boric acid products is less than 1%, and boric acid self-coupling products are not generated), high product purity (the HPLC purity is greater than or equal to 99.99%)and the like, and can be directly applied to OLED terminal materials of devices, and is simple, easy to operate and suitable for large-scale industrial production.
Owner:XIAN RUILIAN NEW MATERIAL CO LTD
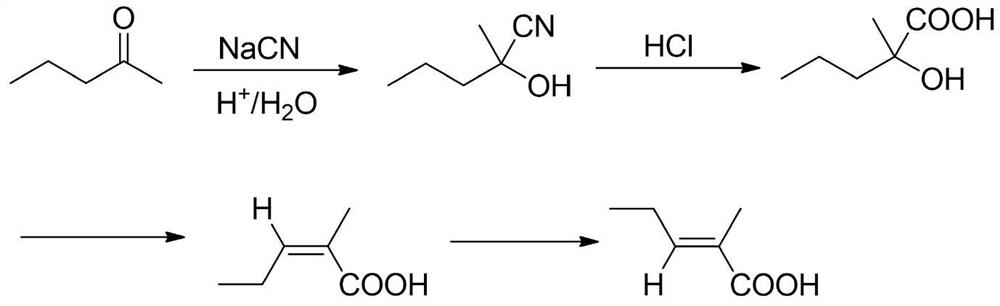
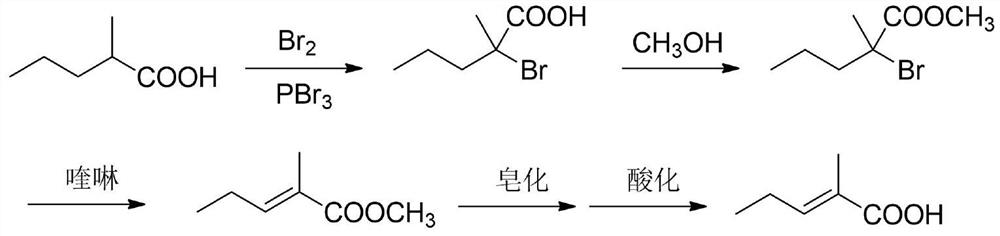
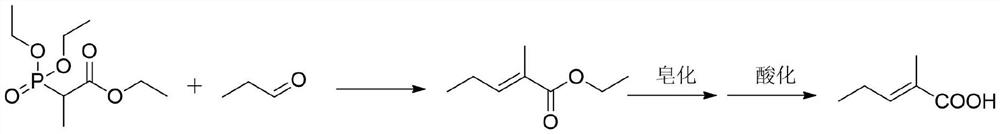
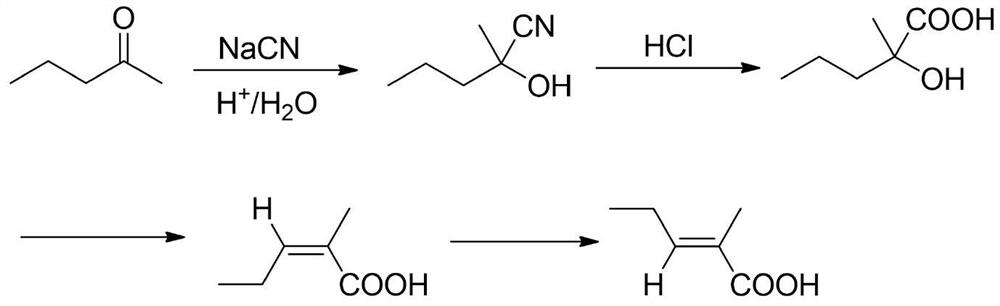
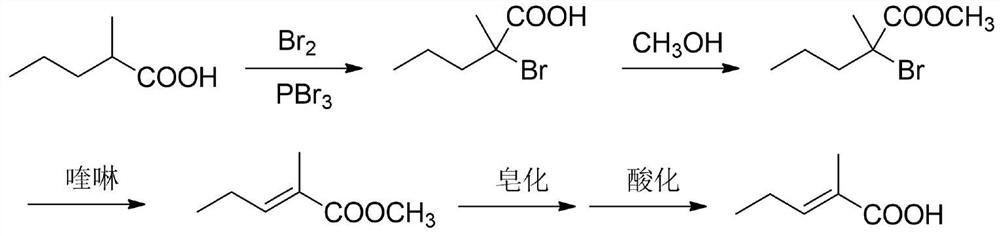
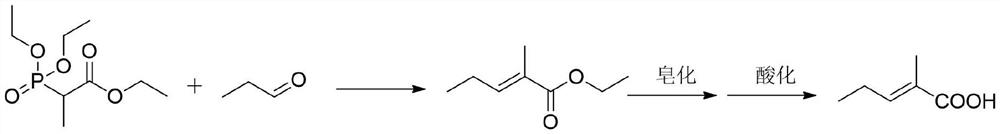
A kind of synthetic method of trans 2-methyl-2-pentenoic acid
ActiveCN113336637BHigh yieldAtom economy is highOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationOrganic chemistry
The present invention adopts the Reppe synthesis method to prepare strawberry acid. First, piperylene, carbon monoxide and water are used as raw materials, and noble metal rhodium salt and organic phosphine ligand are used as catalysts to obtain the cis-trans iso-iso of 2-methyl-3-pentenoic acid. The intermediate product is then isomerized to generate trans-2-methyl-2-pentenoic acid, i.e. trans-strawberry acid.
Owner:SHANDONG NHU PHARMA +1
A method for preparing furfural and two-step preparation of levulinic acid after fractional treatment of biomass
ActiveCN104292193BHigh sugar recoveryHigh yieldOrganic compound preparationCarboxylic compound preparationChemical structureFractionation
The invention provides a method for grading biomass, preparing furfural and preparing levulinic acid (LA) through two steps. The method comprises the following steps: processing a biomass raw material by using an acid / organic solvent combination system to separate lignose, hemicellulose and cellulose, converting hemicellulose into xylooligosaccharide, and converting cellulose into amorphous cellulose; extracting xylooligosaccharide by water, and converting xylooligosaccharide into furfural; and carrying out two-step acid hydrolysis on amorphous cellulose-based residues to make amorphous cellulose in residues converted into 5-HMF (5-hydroxymethylfurfural), introducing the 5-HMF to a second reactor with acidic environment, and carrying out acid hydrolysis to generate the LA. The method has the advantages of extremely high recovery rate / yield of sugar, FF and LA, few side reactions, high recovery rate and small chemical structure change of lignin, less water consumption, fast reaction, low requirements on a reactor, recycling of all reagents, and environmental protection.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
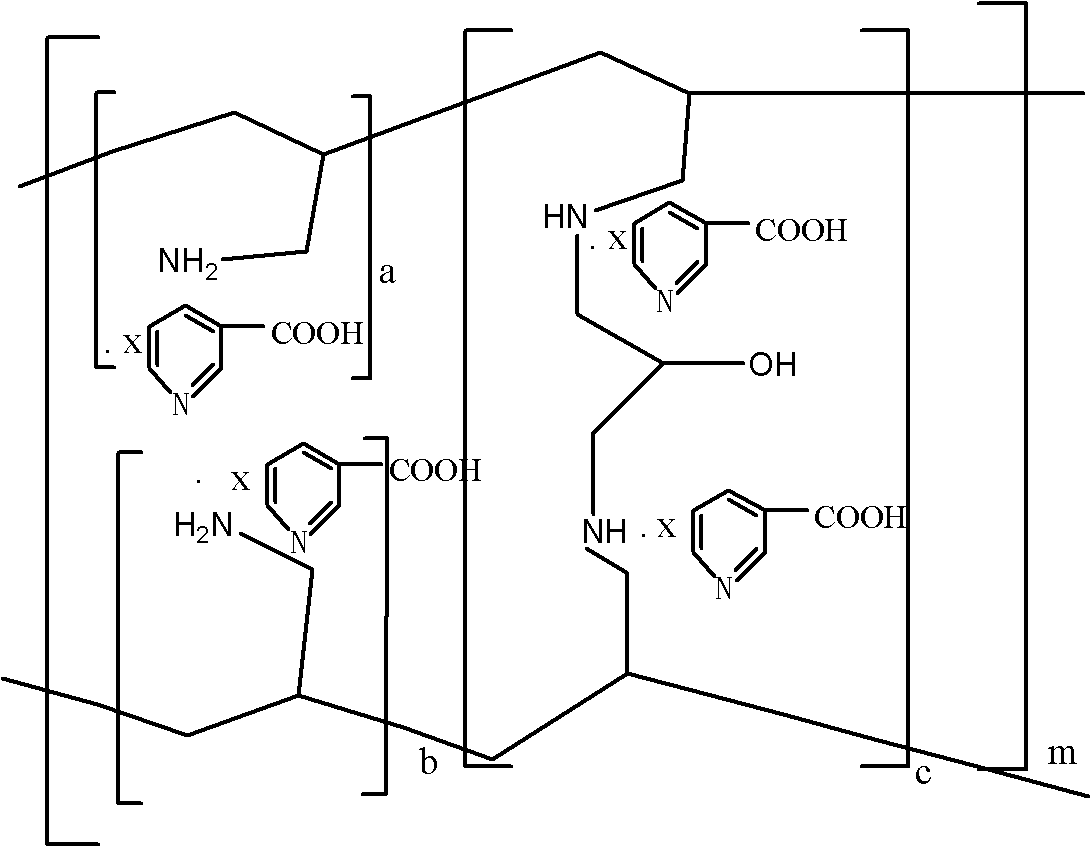
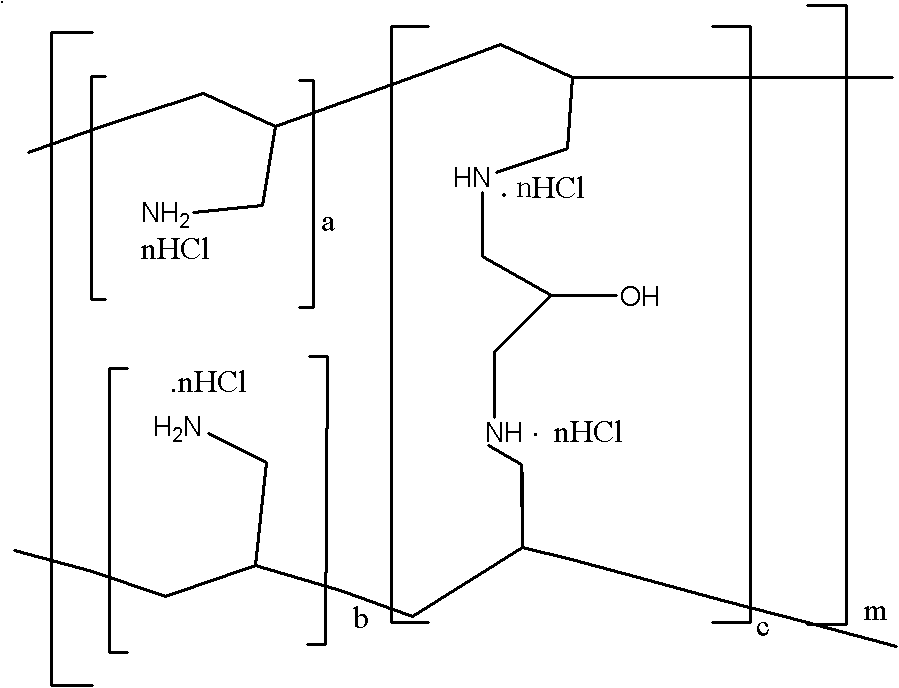
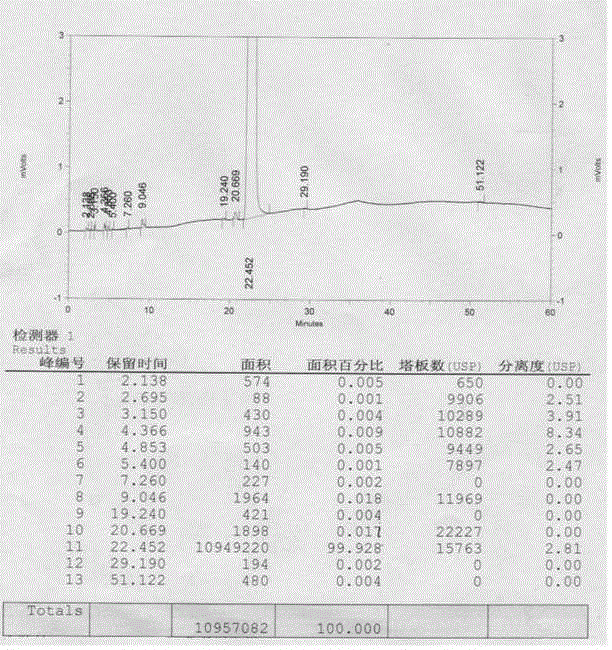
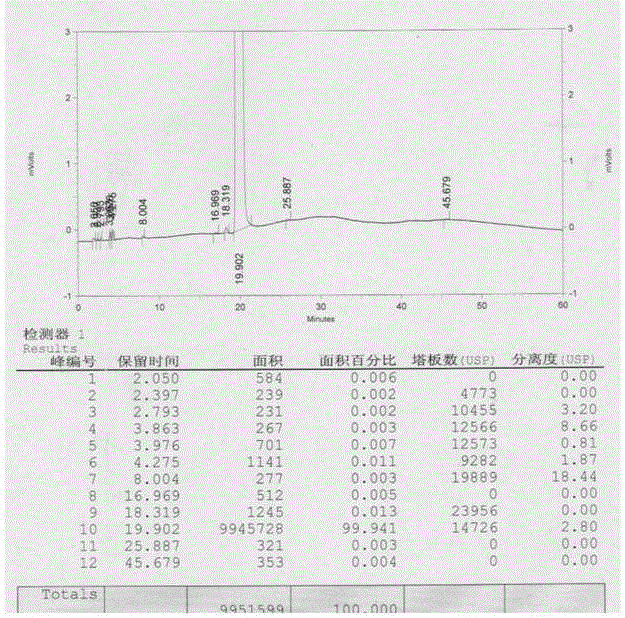
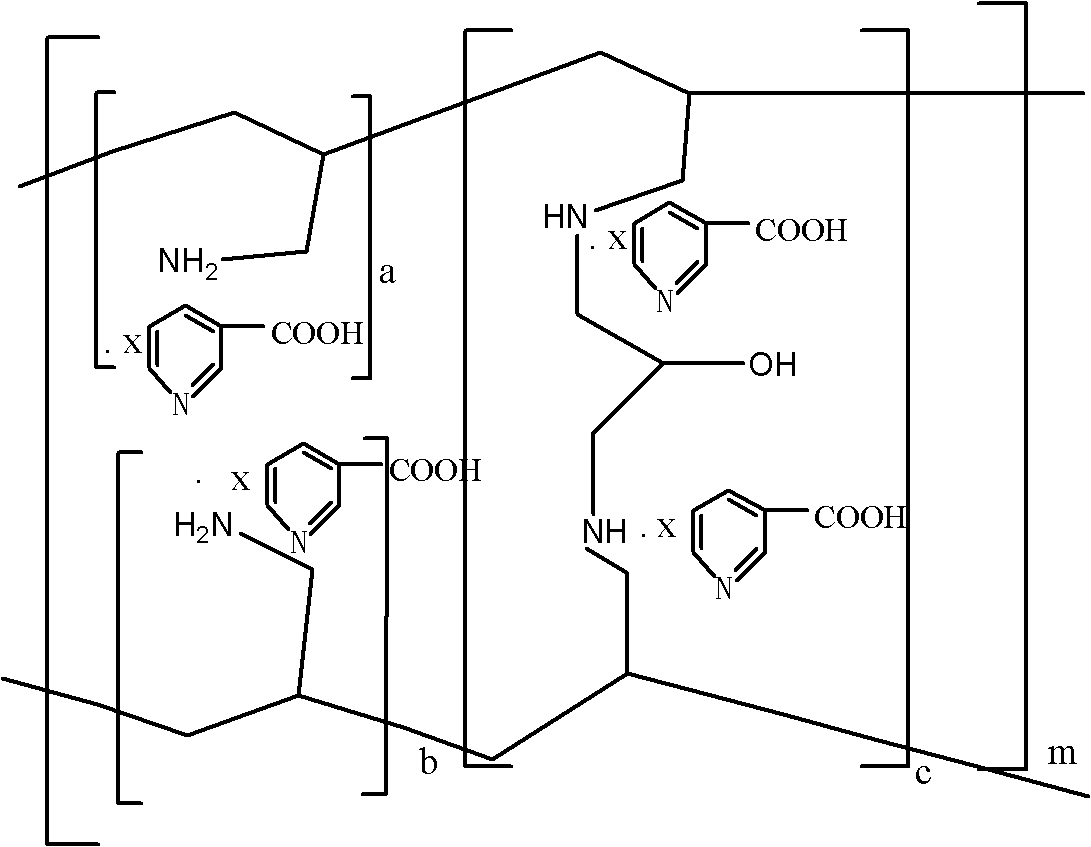
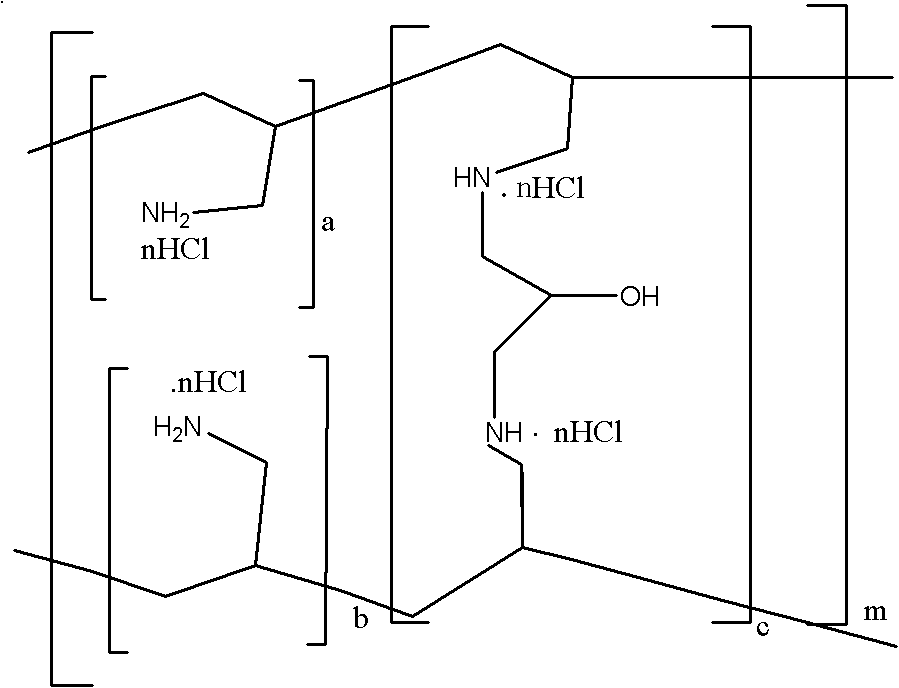
Sevelamer niacin, preparation method and application
InactiveCN102993348BOvercome the disadvantages of excessive chloride ion contentReduce doseOrganic active ingredientsMetabolism disorderNiacinTherapeutic effect
The invention provides sevelamer niacin, a preparation method and an application. The sevelamer has a structural formula I shown as follows; wherein in the structural formula I, the ratio of (a+b): c is 45: 1 to 2: 1, m represents an integer which is not the zero or negative number, each x independently represents 0 or 1, and each x can not be 0 at the same time; and the proportion of the sevelamer niacin is 2.00-2.50. The sevelamer niacin has similar therapeutic effect with sevelamer hydrochloride under the condition that the usage amount of sevelamer niacin is 50% of that of sevelamer hydrochloride.
Owner:NEW FOUNDER HLDG DEV LLC +2
Synthesis method of neotame
ActiveCN104177473BHigh selectivityExtended service lifePeptide preparation methodsHydrogenOrganic solvent
The invention relates to a synthesis method of neotame. The method comprises the following steps: (1) adding aspartame and 3,3-dimethyl-butyl aldehyde to an organic solvent in a vacuum state, reacting at the reaction temperature of 20-50 DEG C for 5-24 hours, carrying out concentration and separation at 0-50 DEG C after reaction is ended, concentrating to obtain white paste, adding deionized water to devitrify, thus obtaining a neotame intermediate imine in a centrifuging manner; (2) adding imine and an organic solvent to a hydrogenation reactor, introducing hydrogen until the pressure is 0.1-0.5MPa, adding a catalyst, reacting at the temperature of 35-40 DEG C for 5-30 hours, filtering and removing the catalyst, concentrating the filtrate and removing an organic solvent, and recrystallizing and purifying by water, methanol or ethanol, so as to obtain the neotame. According to the synthesis method, the defects in the prior art are overcome, high-purity imine is generated, the activity of the catalyst is reduced, the selectivity of the catalyst on the imine is increased, the service life of the catalyst is prolonged, the reaction impurity is reduced, and the product purity is improved.
Owner:SHANDONG BENYUE BIOTECH
The preparation method of 7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid
The invention discloses a preparation method of 7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid, and relates to the technical field of preparation of pharmaceutical intermediates. The method includes the steps that under the protection of nitrogen, 7-aminocephalosporanic acid, a silane reagent and an imidazole catalyst are stirred for 0.5-2 h at 30-35 DEG C in a water-soluble organic solvent; then, the mixture is cooled to 0-5 DEG C, methanesulfonic acid, trimethyl borate and methyl alcohol are added, a methoxylation reaction is conducted with the temperature controlled to be 0-5 DEG C, and 7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid is obtained, wherein the silane reagent is hexamethyldisilazane or trimethylchlorosilane or N,O-bis(trimethylsilyl)acetamide or dimethoxydimethylsilane. According to the method, the product purity is high, the impurity content is low, operation is easy and convenient, refining steps are simplified, production cost is reduced, and the method is suitable for industrial production.
Owner:湖北凌晟药业股份有限公司
A kind of preparation method of n(2)-l-alanyl-l-glutamine
ActiveCN103626839BPrice stabilityMarket sales trend ups and downsPeptide preparation methodsL-alanyl-l-glutamineChloride
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of N(2)-L-alanyl-L-glutamine. The preparation method of the N(2)-L-alanyl-L-glutamine comprises the following steps of (1) preparing L-phthaloyl-alanyl chloride; (2) preparing phthaloyl-L-alanyl-L-glutamic acid; (3) preparing phthaloyl-L-alanyl-L-glutamic acid anhydride; (4) preparing phthaloyl-L-alanyl-L-glutamine; (5) preparing an N(2)-L-alanyl-L-glutamine crude product; (6) preparing an N(2)-L-alanyl-L-glutamine refined product. The product obtained through the final deprotection process of the preparation method as a pilot plant test or a production scale process route is higher in purity; the liquid-phase purity of the product is higher than 99.9% through primary purification, and the product is low in impurity content.
Owner:JINAN CHENGHUI SHUANGDA CHEM
A kind of preparation method of 5'-adenylic acid
ActiveCN107602648BImprove conversion rateFair priceSugar derivativesSugar derivatives preparationAdenosinePhosphoric acid
The invention discloses a preparation method of 5'-adenylic acid. The preparation method of 5'-adenylic acid comprises the following steps: performing synthesis reaction on powdered adenosine and polyphosphoric acid which serve as raw materials under the condition of no solvent, adding pure water after the synthesis reaction to perform hydrolysis, and refining the crude product obtained by the hydrolysis, wherein the mass ratio of the powdered adenosine to the polyphosphoric acid to the pure water is 1:(2-4):(2-4). The preparation method of 5'-adenylic acid has the following advantages: the solvent-free synthesis method is adopted, the reaction impurities are few, the condition is mild and pollution is avoided; the product yield is 90 percent or above, the product content is more than 99 percent and the conversion rate of the raw materials is high; and the operation steps are simple, the raw materials are wide in source and the price is moderate, so the preparation method of 5'-adenylic acid can be popularized and implemented on a large scale.
Owner:江苏香地化学有限公司
Sevelamer niacin, preparation method and application
InactiveCN102993348AOvercome the disadvantages of excessive chloride ion contentReduce doseOrganic active ingredientsMetabolism disorderTherapeutic effectNiacin
The invention provides sevelamer niacin, a preparation method and an application. The sevelamer has a structural formula I shown as follows; wherein in the structural formula I, the ratio of (a+b): c is 45: 1 to 2: 1, m represents an integer which is not the zero or negative number, each x independently represents 0 or 1, and each x can not be 0 at the same time; and the proportion of the sevelamer niacin is 2.00-2.50. The sevelamer niacin has similar therapeutic effect with sevelamer hydrochloride under the condition that the usage amount of sevelamer niacin is 50% of that of sevelamer hydrochloride.
Owner:NEW FOUNDER HLDG DEV LLC +2
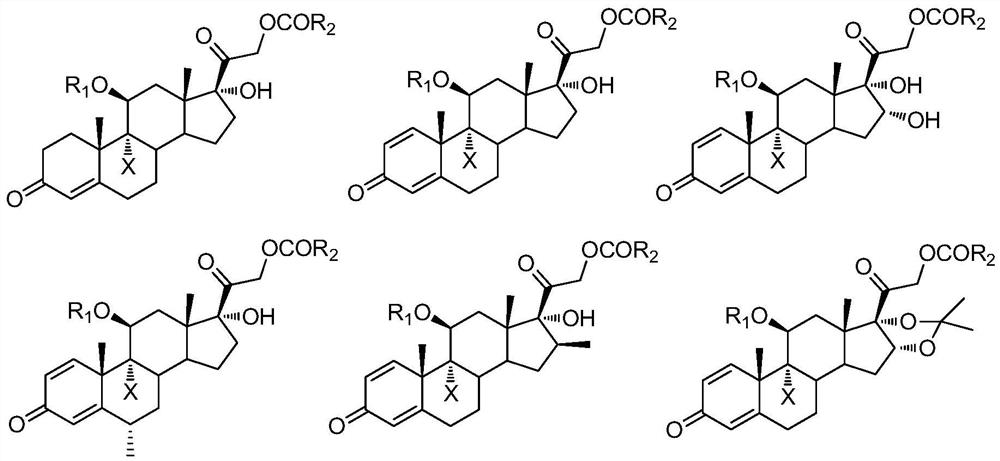
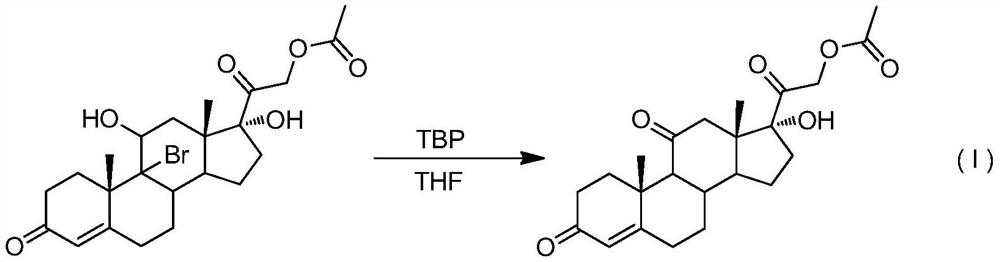
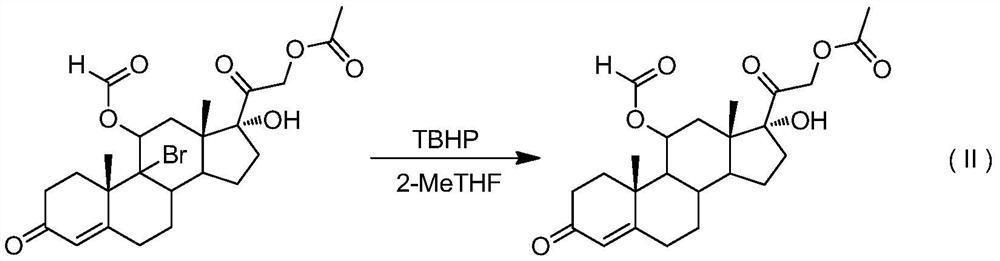
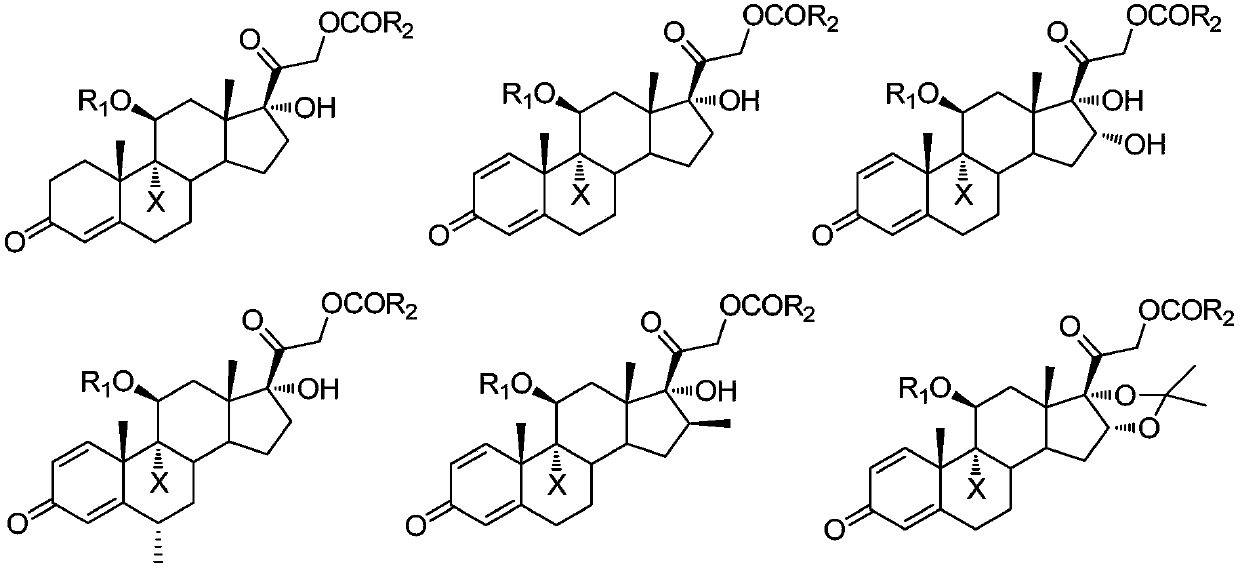
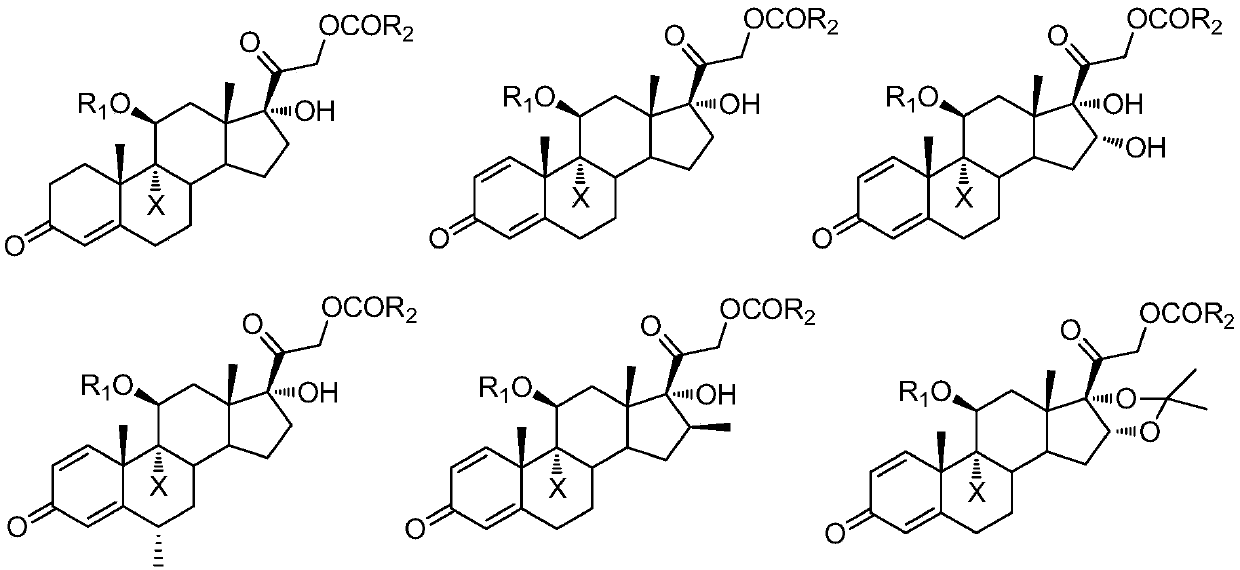
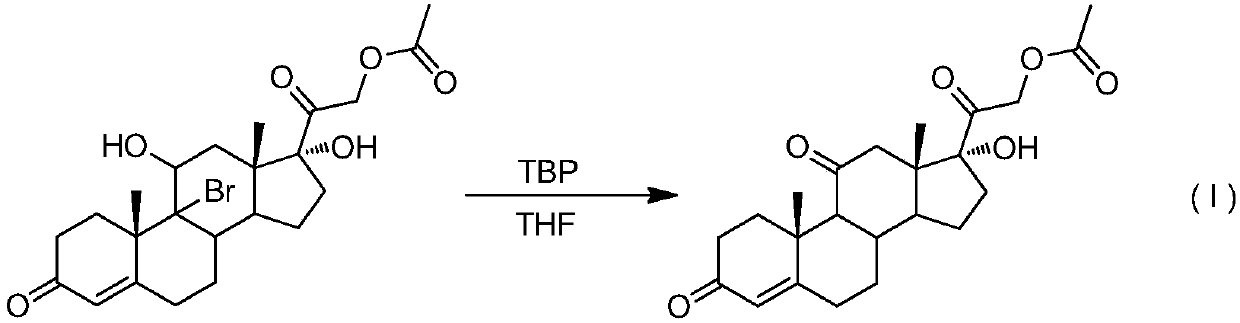
A kind of preparation method of 9-position dehalogenation of 9-halogenated steroid hormone compound
ActiveCN111333690BThree wastes less pollutionEasy to operateSteroidsBulk chemical productionOrganic solventFatty acid
The invention discloses a method for dehalogenating the 9-position of a steroid hormone compound. The method comprises: dissolving a 9-halogenated-11-hydroxy steroid compound or a 9-halogenated-11-hydroxy ester steroid compound in an organic In the solvent, after heating up to a certain temperature, slowly add an appropriate amount of dehalogenation reagent, and detect after a period of reaction. After the reaction is complete, concentrate under reduced pressure to remove part of the solvent, cool down to crystallize or add water to crystallize, precipitate a solid, and filter to obtain the target product 11 ‑carbonyl steroids or 11‑hydroxyester steroids. The invention has simple operation, less reaction impurities, high yield, avoids the use of metal dehalogenating agent and mercapto fatty acid, reduces pollution of three wastes, and is suitable for industrial production.
Owner:AURISCO PHARMACEUTICAL CO LTD
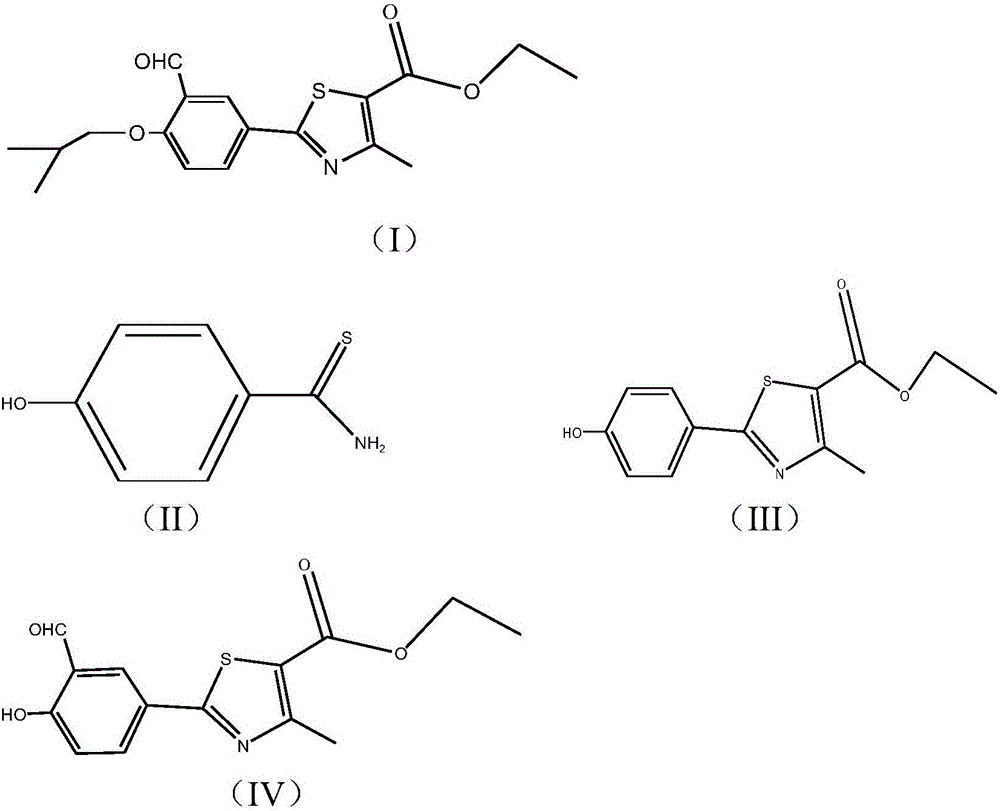
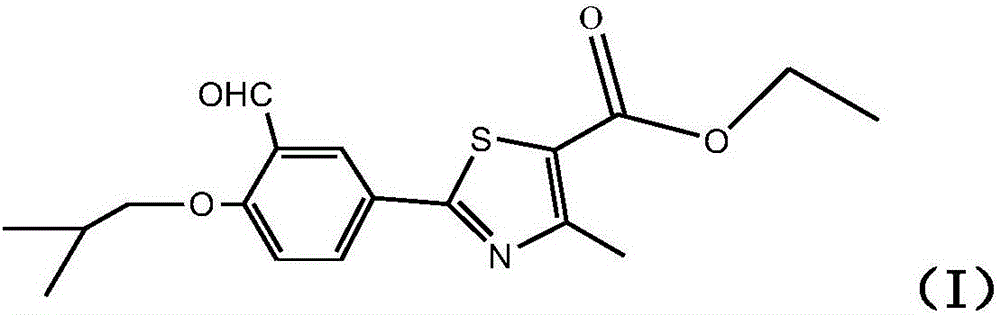
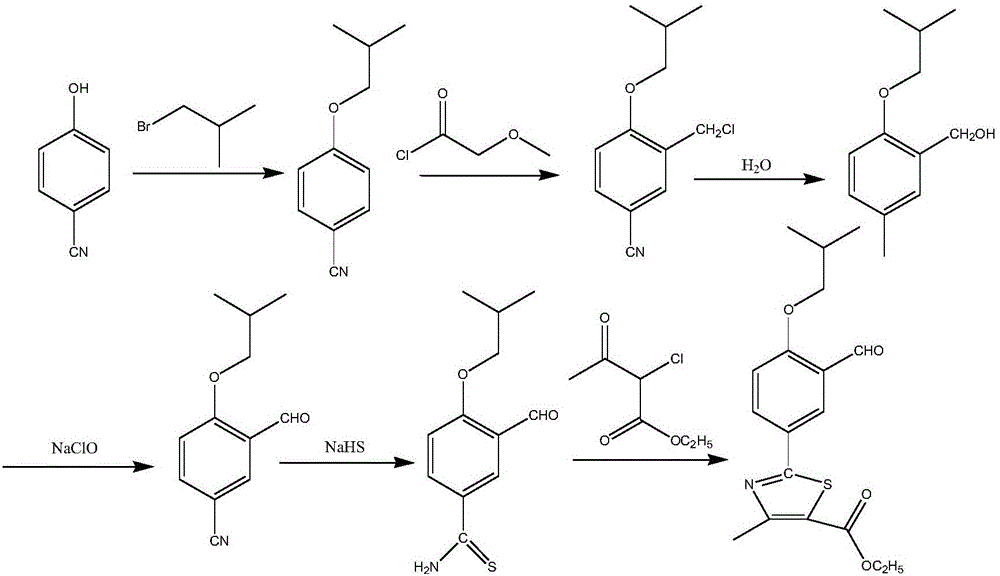
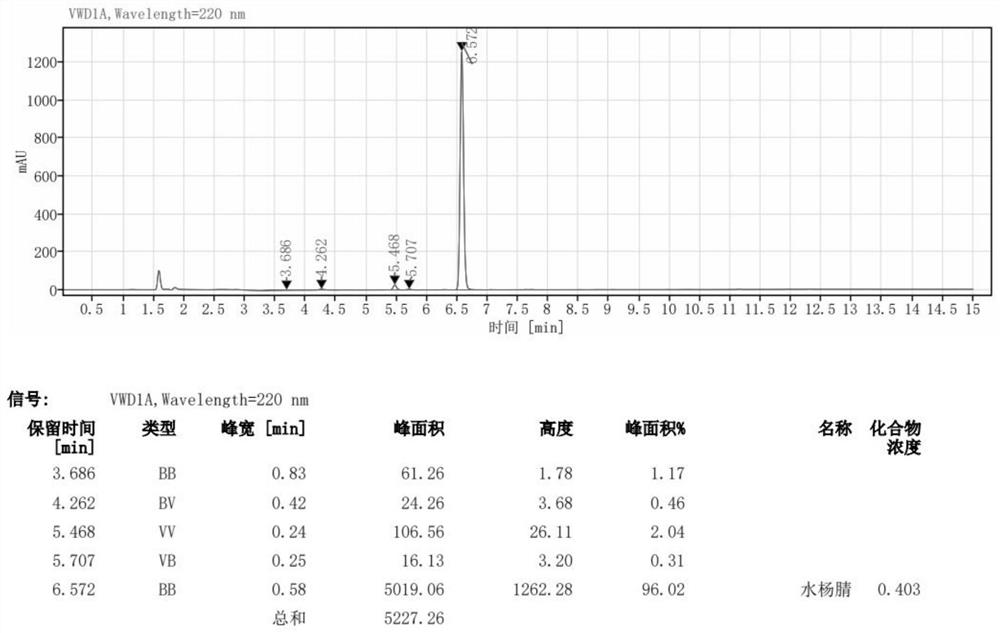
The synthetic method of 2‑(3‑formyl‑4‑isobutoxyphenyl)‑4‑methylthiazole‑5‑ethyl carboxylate
ActiveCN104529935BThe synthesis method is simpleGuaranteed responseOrganic chemistryThiazoleFormylation reaction
The invention provides a method for synthesizing high-purity ethyl 2-(3-aldehyde-4-isobutyloxyphenyl)-4-methylthiazole-5-formate. The method comprises the following steps of carrying out thioacylation reaction on p-cyanophenol and thioacetamide as starting materials to obtain 4-hydroxythiobenzamide (II), directly carrying out thiazole reaction on the reaction product which is not separated and separating to obtain ethyl 2-(4-hydroxyphenyl)-4-methyl-5-thiazole formate (III); carrying out formylation reaction on the compound as shown in the formula (III) to obtain ethyl 2-(3-carbaldehyde-4-hydroxyphenyl)-4-methyl-5-thiazole formate (IV), directly carrying out isobutylation reaction on the reaction product which is not separated to obtain ethyl 2-(3-aldehyde-4-isobutyloxyphenyl)-4-methylthiazole-5-formate (I) of which the purity is equal to or greater than 99% and the content is equal to or greater than 99%. The production process is optimized and thus the quality of the product is greatly improved and the high yield is achieved.
Owner:ZHEJIANG HUAYI PHARMA CO LTD OF HANGZHOU HUADONG PHARMA GRP +1
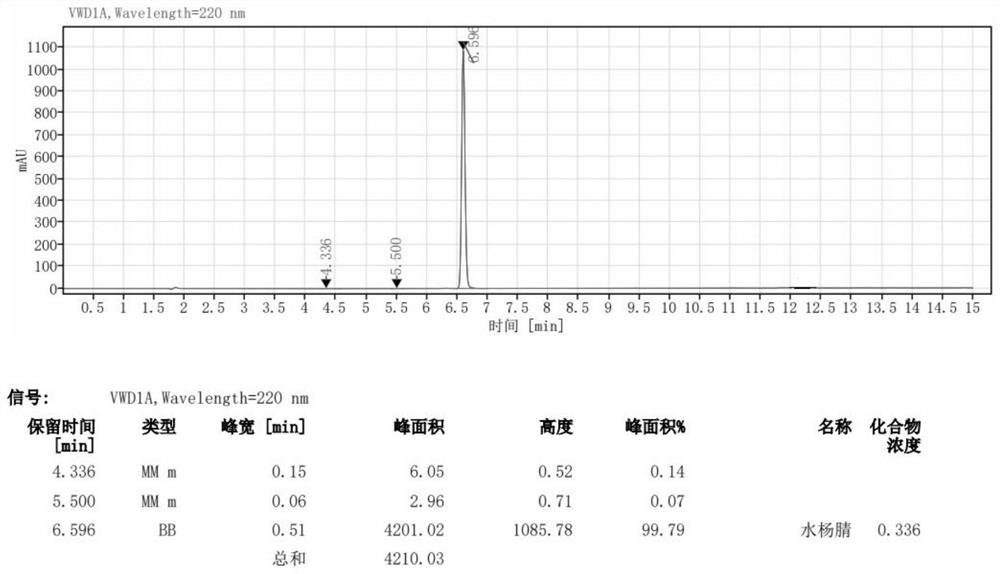
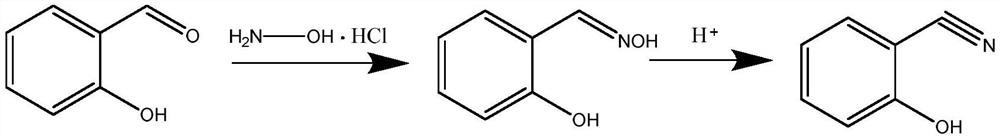
Method for preparing 2-hydroxy-benzonitril by adopting micro-flow field technology
ActiveCN113248402AEasy to operateLow costCarboxylic acid nitrile preparationOrganic compound preparationSalicylaldehydeHydroxylamine
The invention discloses a method for preparing 2-hydroxy benzonitrile (o-hydroxy benzonitrile) by adopting a micro-flow field technology, which comprises the following steps: (1) dissolving hydroxylamine hydrochloride in a solvent, adding salicylaldehyde, and fully stirring and uniformly mixing to obtain a reaction solution; and (2) pumping the uniformly mixed reaction liquid into the micro-channel reactor by adopting single-strand feeding, and carrying out one-step reaction to obtain a 2-hydroxy-benzonitrile crude product. Compared with a traditional preparation method of the 2-hydroxy-benzonitrile, the method has the advantages that the use of a dehydrating agent is avoided, the reaction time is short, the conversion rate of the 2-hydroxy-benzonitrile is high, the operation is simple, the safety is high, and the method is suitable for industrial production.
Owner:NANJING ADVANCED BIOLOGICAL MATERIALS & PROCESS EQUIP INST CO LTD +1
Large-scale preparation method and application of polypeptide
PendingCN111349147AImprove reaction efficiencyLow costPeptide-nucleic acidsPeptide/protein ingredientsAmino acid synthesisHexahydropyridine
The invention provides a large-scale preparation method and application of polypeptide. The method comprises the following steps of: taking 2-chloro-triphenylchloride resin as a starting raw material,connecting a first Fmoc protective amino acid, and sealing by using a sealing reagent; taking the Fmoc protective amino acid as a monomer, taking a hexahydropyridine solution as a deprotection reagent, and sequentially connecting amino acids one by one under the action of a condensing agent and an alkaline condition to synthesize the polypeptide; cutting the polypeptide, and concentrating to obtain a polypeptide crude product; and purifying the crude product to obtain a polypeptide product. The method can be used for preparing pure peptides of a hectogram grade and a kilogram grade, the problem of gram-scale production of the polypeptide as a raw material medicine is solved, and a simple and effective way is provided for preparing a reaction biological material.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Oxidase whole-cell catalyst and method for preparing high-optical-purity R-type 1, 3-butanediol by using oxidase whole-cell catalyst
PendingCN114480459ALess impurities in the reactionLow costBacteriaMicroorganism based processesPtru catalystOxidative enzyme
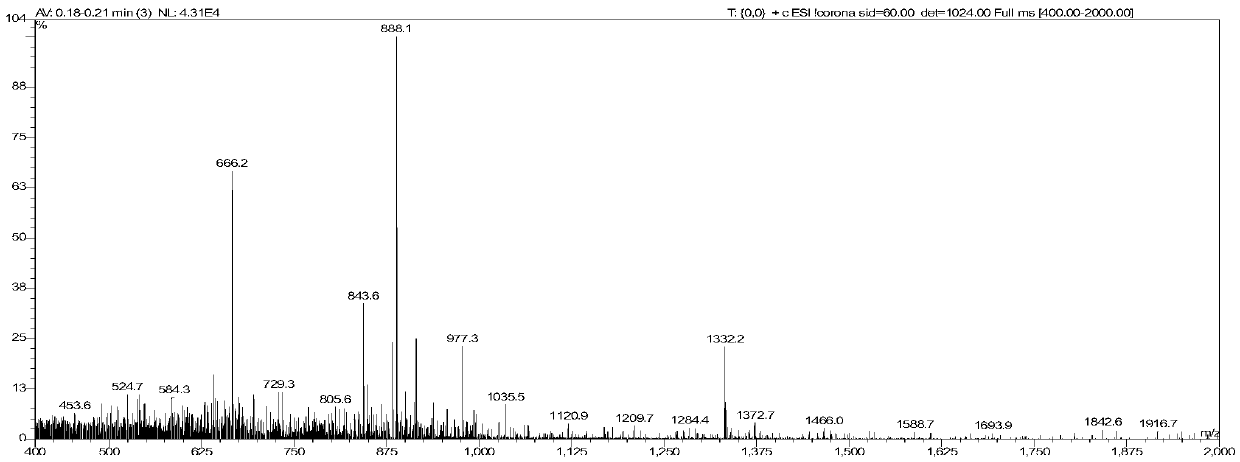
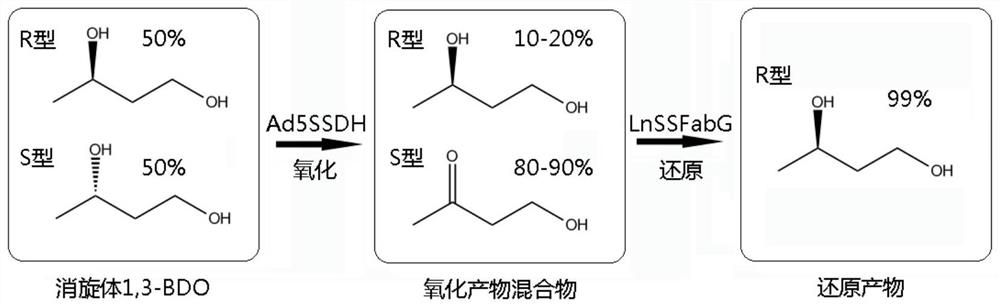
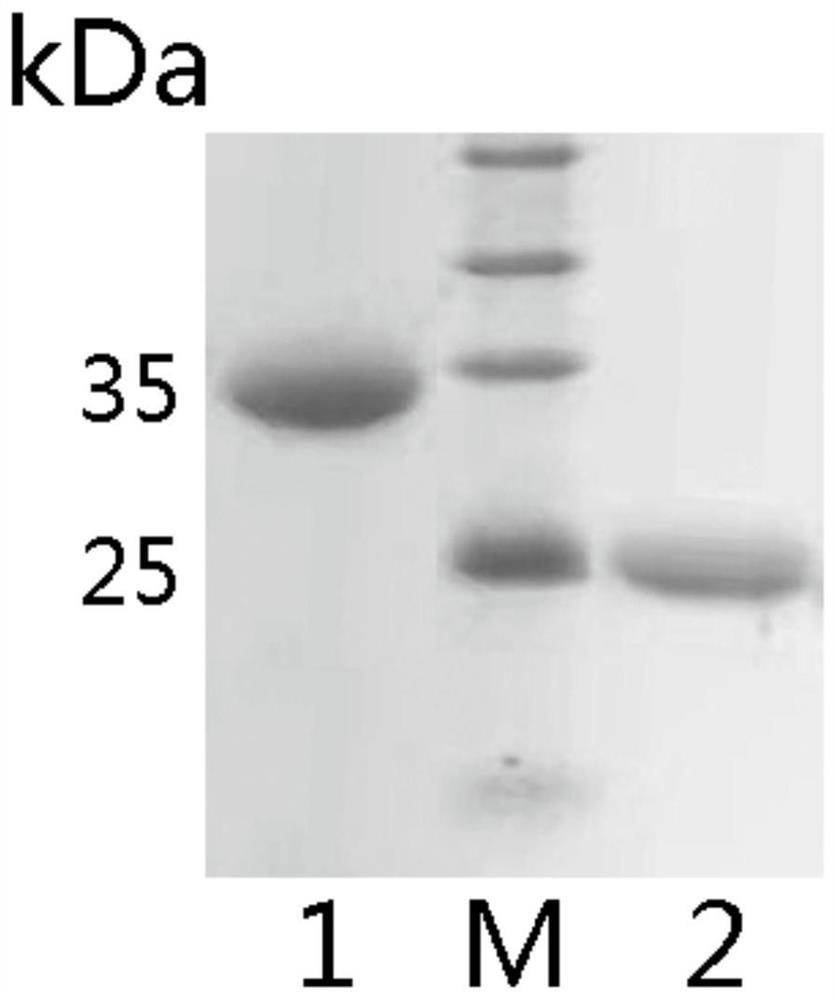
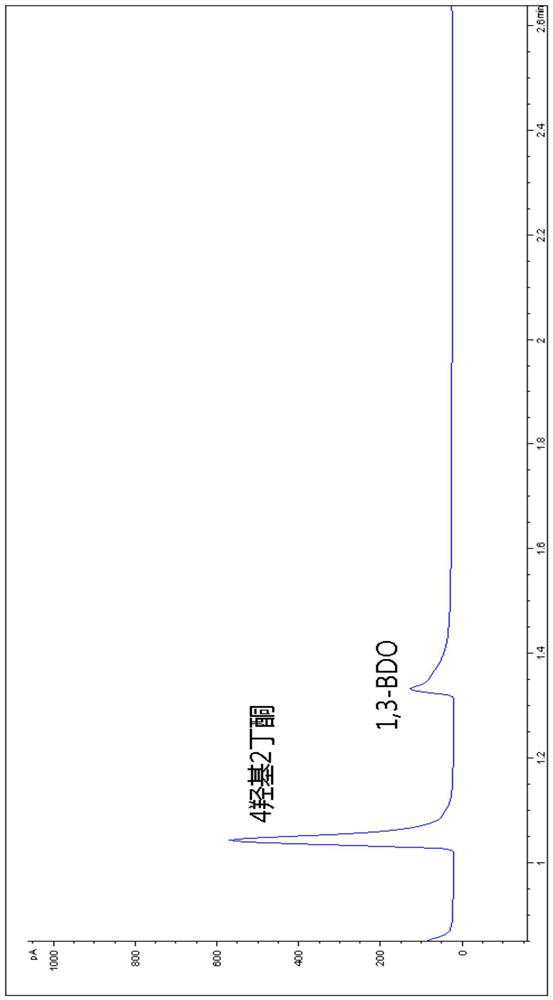
The invention relates to an oxidase whole-cell catalyst and a method for preparing high-optical-purity R-type 1, 3-butanediol by using the oxidase whole-cell catalyst. The invention relates to the technical field of preparation of R-type 1, 3-butanediol through biological catalysis. The invention provides an oxidase whole-cell catalyst. A preparation method of the oxidase whole-cell catalyst comprises the following steps: after an Ad5SSDH gene is subjected to PCR (Polymerase Chain Reaction) amplification, inserting the Ad5SSDH gene into a pET30a carrier to obtain a plasmid pET30Ad5SSDH; the method comprises the following steps: transforming a plasmid pET30Ad5SSDH into recipient bacteria escherichia coli BL21 (DE3), so as to obtain a genetically engineered bacterium with high oxidase yield; and purifying the high-yield oxidase gene engineering bacteria to obtain the oxidase whole-cell catalyst. According to the method disclosed by the invention, the enzymatic production of R-1, 3-BDO is completely carried out by adopting a biological enzyme technology, and compared with a chemical method, the pollution to the environment in the production process is reduced. The feeding concentration reaches 6%, the conversion time is 36 hours, the conversion rate is 80%, and the chiral purity is 99.2%.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Synthesis method of trans-2-methyl-2-pentenoic acid
ActiveCN113336637AAvoid Yield ProblemsLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationPtru catalyst
The invention adopts a Reppe synthesis method to prepare 2-methyl-2-pentenoic acid. The method comprises the following steps: firstly, by taking m-pentadiene, carbon monoxide and water as raw materials, and taking precious metal rhodium salt and an organic phosphine ligand as catalysts, preparing a cis-trans isomeric intermediate of 2-methyl-3-pentenoic acid, and then carrying out isomerization reaction on the obtained intermediate product to generate trans-2-methyl-2-pentenoic acid, namely trans strawberry acid.
Owner:SHANDONG NHU PHARMA +1
9-site dehalogenation preparation method of 9-halogenated steroid hormone compound
ActiveCN111333690AThree wastes less pollutionEasy to operateSteroidsBulk chemical productionFatty acidHalogenated steroids
The invention discloses a 9-site dehalogenation method of a steroid hormone compound. The method comprises the following steps of: dissolving a 9-halogenated-11-hydroxy steroid compound or a 9-halogenated-11-hydroxy ester steroid compound into an organic solvent; heating to a certain temperature, slowly adding a proper amount of a dehalogenation reagent, carrying out a reaction for a period of time, carrying out detection, carrying out reduced pressure concentration to remove a part of the solvent after the reaction is completed, carrying out cooling crystallization or crystallization by adding water, separating out a solid, and filtering to obtain the target product that is a 11-carbonyl steroid compound or a 11-hydroxy ester steroid compound. The method has the advantages of simple operation, few reaction impurities, high yield, capability of avoiding of the use of a metal dehalogenation agent and mercaptofatty acid, reduction of three-waste pollution, and suitableness for industrialproduction.
Owner:AURISCO PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com