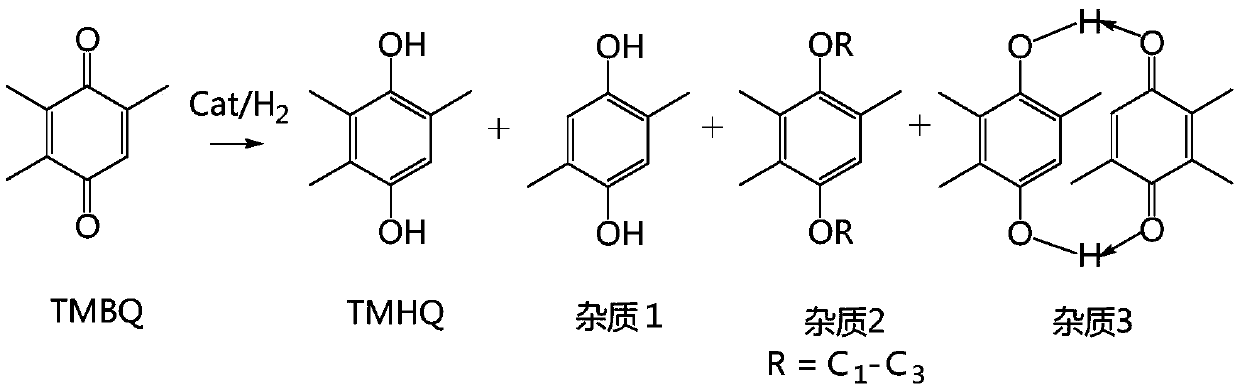
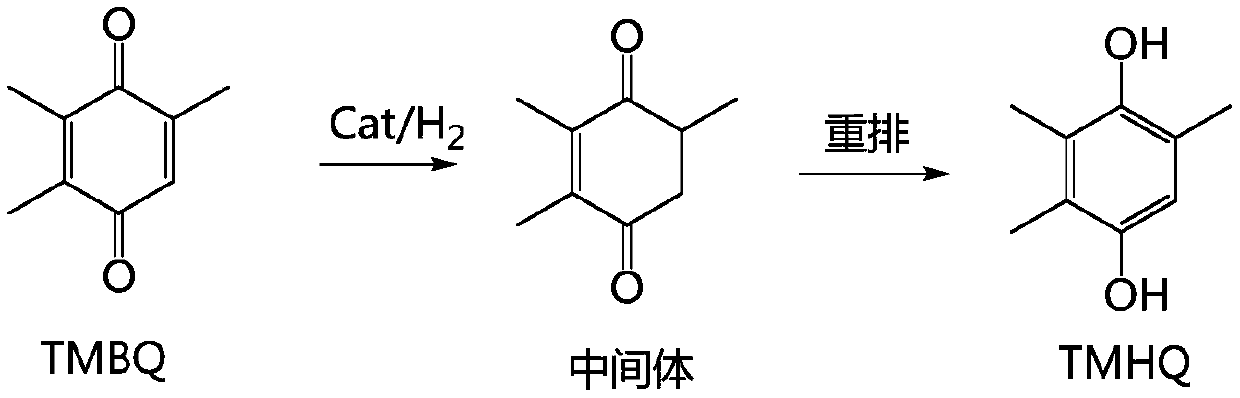
Synthesis method and device of 2,3,5-trimethylhydroquinone
A trimethylhydroquinone and synthesis method technology, applied in the field of fine organic chemical synthesis, can solve the problems of large recovery loss, troublesome solvent treatment, and further improvement of hydrogenation reaction selectivity, so as to improve the hydrogenation reaction selectivity, Reduce the hydrogenation reaction activity and change the effect of the hydrogen absorption capacity of the solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
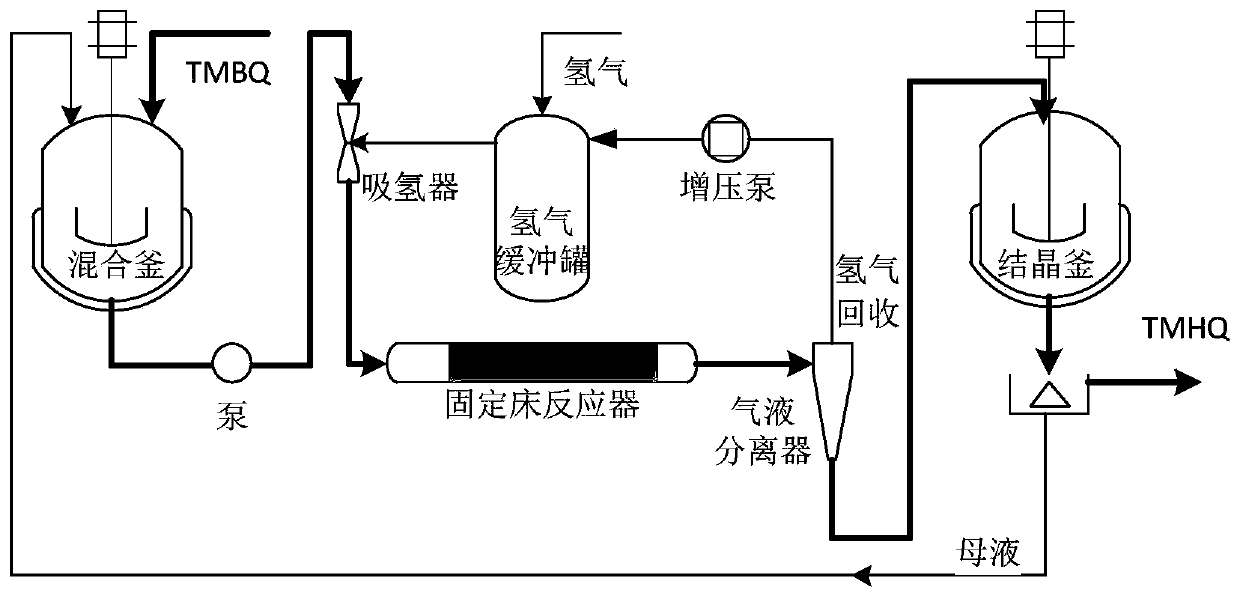
[0054] Put 1000g of methanol: benzene = 1:1 (W:W) mixed solvent in a 2000mL reaction kettle, TMBQ200g, stir, preheat to 50°C, pump into the hydrogen absorber, inhale hydrogen for mixing, and then enter the 20g A fixed-bed reactor (tubular reactor) with a palladium-carbon catalyst content of 2%, and the temperature is kept at 50°C. The residence time of the material in the fixed bed is 10min. After the reaction, it enters the crystallization tank for cooling and crystallization, and the product TMHQ is centrifugally filtered: 202.70g. Tracking detection shows that the conversion rate: 99.95%, selectivity: 99.92%, and yield: 99.87%. Purity: 99.87%, total impurities: 0.13%.
Embodiment 2-20
[0056] The difference between Examples 2-20 and Example 1 lies in the selection of different solvents, different proportions and reaction temperatures, and other conditions are the same as in Example 1. All the experimental results are summarized in Table 1.
Embodiment 21
[0058] In the 2000mL reactor, drop into methanol 1000g, TMBQ200g, stir, preheat to 50 ℃, pump into the hydrogen absorber, inhale hydrogen and mix, then enter into the palladium-carbon catalyst fixed-bed reactor that is 2% that 20g content is housed (tube type reactor), the temperature was maintained at 65°C. The residence time of the material in the fixed bed is 10min. After the reaction, it enters the crystallization tank for cooling and crystallization, and the product TMHQ is filtered out by centrifugation: 201.70g. Tracking detection shows that the conversion rate: 99.50%, selectivity: 99.00%, yield: 98.51%, Purity: 99.00%, total impurities: 1.00%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com