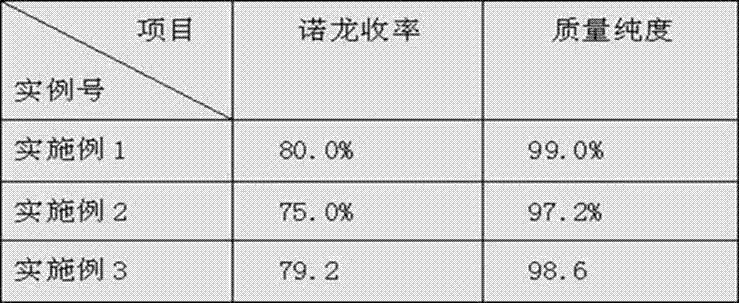
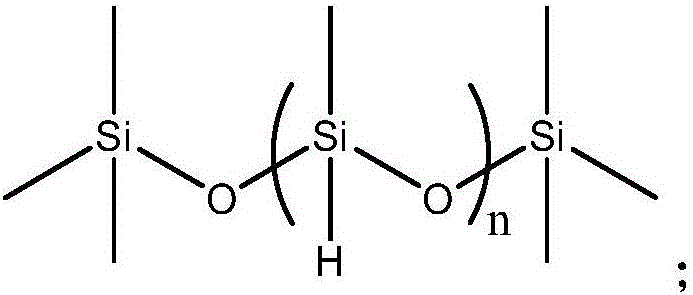
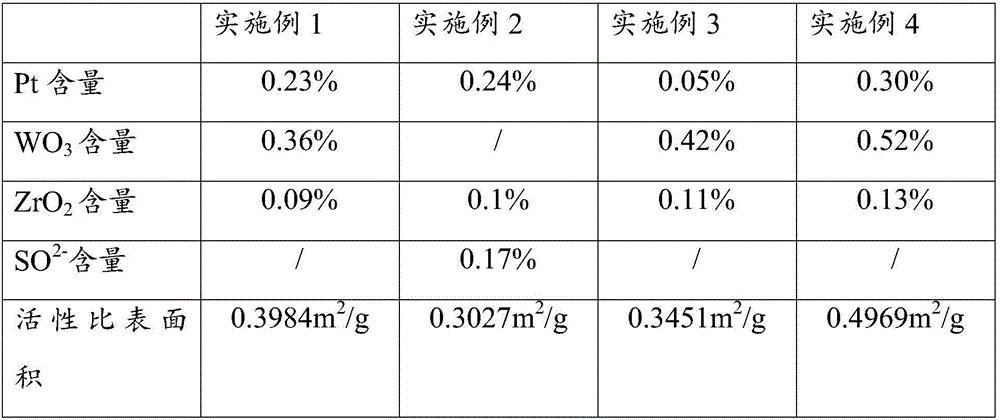
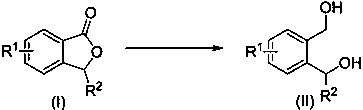Patents
Literature
46results about How to "Strong reaction selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
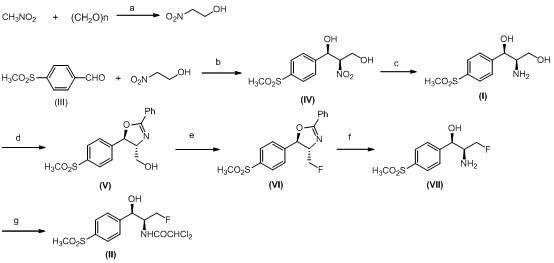
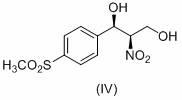
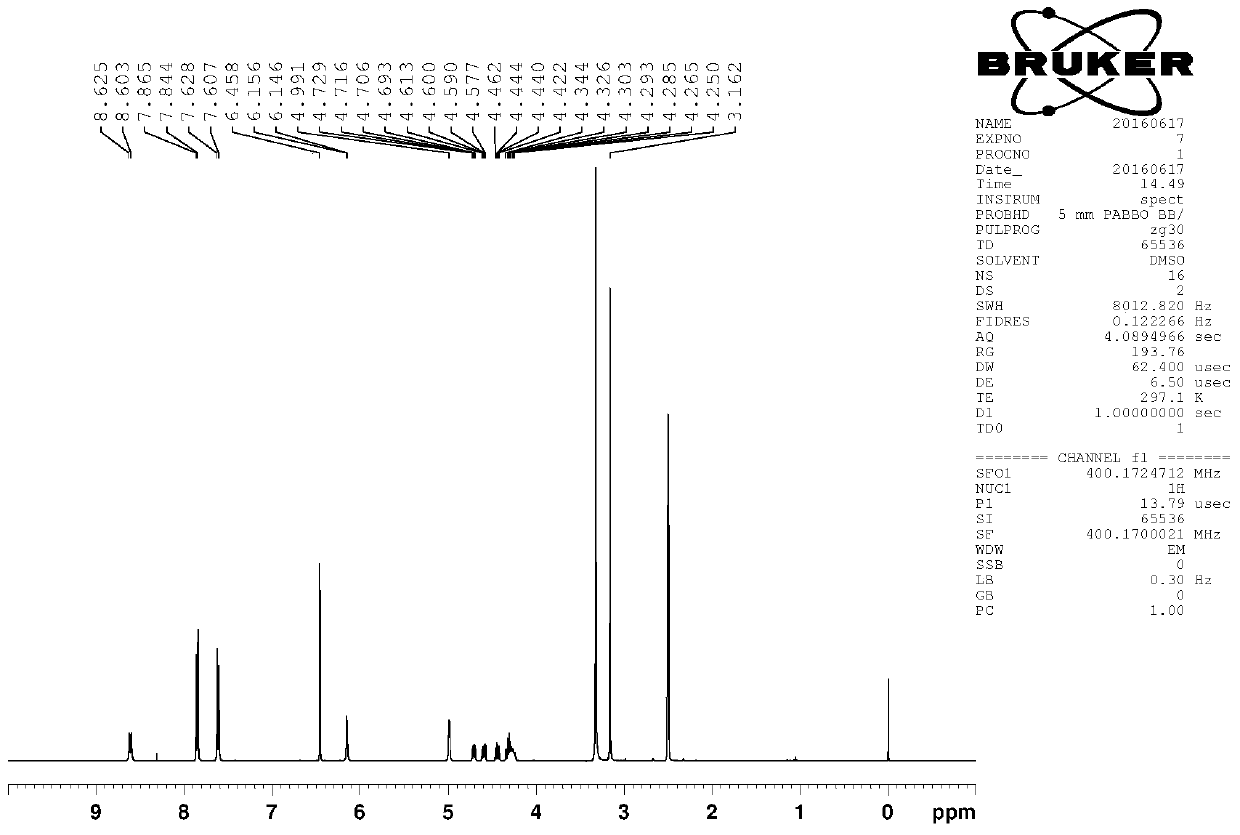
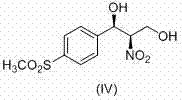
Method for analyzing (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol as intermediate of florfenicol
ActiveCN101941927AEasy to manufactureHigh yieldOrganic chemistryOrganic compound preparationPtru catalystBenzaldehyde
The invention relates to a method for analyzing (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol as an important intermediate of florfenicol, which is chloramphenicol spectrum antibiotic special for animals. The method comprises the following steps of: performing a reaction of methylsulfonyl benzaldehyde and nitro alcohol at 0-40 DEG C in the presence of a chiral catalyst to obtain (1R, 2R)-2-nitro-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol; and reducing the (1R, 2R)-2-nitro-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol through hydrogen to obtain the intermediate. In the invention, the compound (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol with a high e.e. value is obtained by adopting the chiral catalyst, and the compound has the advantages of strong reaction selectivity, high productivity, simple process, low cost, easy preparation of raw materials and low price, is suitable for industrial production, avoids chiral separation frequently used in industry at present and saves the raw materials and the cost. The compound (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol is used for preparing florfenicol and can reduce the preparation cost.
Owner:MASTEAM BIO TECH
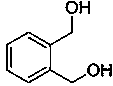
Method for synthesizing o-benzenedicarbinol derivative
ActiveCN108610237AHigh yield preparationSimple processCarboxylic acid nitrile preparationOrganic compound preparationSilane compoundsPhthalide
The invention belongs to the technical field of chemical engineering and particularly relates to a method for synthesizing an o-benzenedicarbinol derivative. Phthalide as a raw material is subjected to a reduction ring-opening reaction under the action of alkali and silane compounds, and o-benzenedicarbinol is prepared. According to the method, raw materials are easy to obtain, the method is simple and convenient to operate, the reaction selectivity is high, the product yield is high, and the reaction condition is mild; the synthesized monosubstituted or polysubstituted o-benzenedicarbinol derivative has high quality and good functional group compatibility.
Owner:FUDAN UNIV
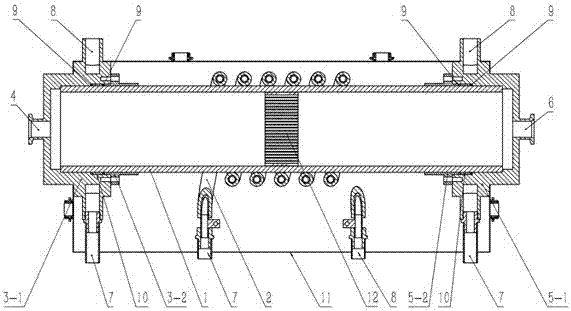
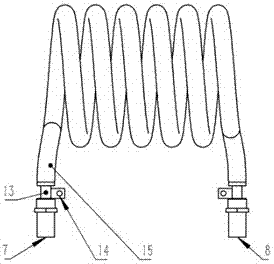
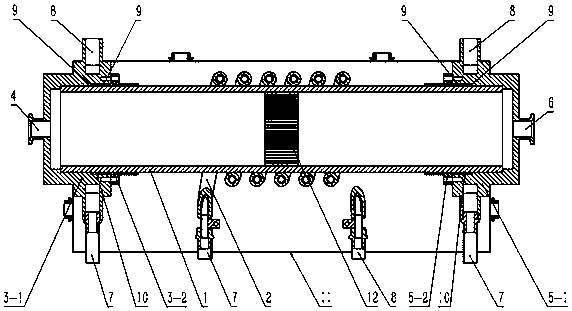
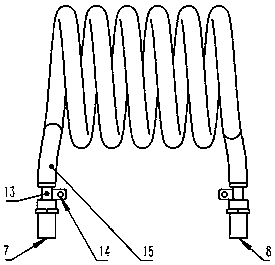
Radio frequency plasma-catalyst synergic effect reaction device
InactiveCN106975349AImprove energy efficiencyStrong reaction selectivityPhysical/chemical process catalystsDispersed particle separationChemical reactionHigh energy
The invention discloses a radio frequency plasma-catalyst synergic effect reaction device, which comprises components such as a non-balance plasma reaction chamber, a hollow tubular metal spiral coil with a cooling passage, a catalysis body with a catalyst, an air inlet sealing end part and air inlet end sealing flange, an exhaust sealing end part and exhaust end sealing flange, an electromagnetic shielding cover and the like. The radio frequency plasma-catalyst synergic effect reaction device belongs to a reaction device arranged by aiming at the inductance coupling radio-frequency plasma and catalyst synergic effect; the non-balance plasma reaction chamber made of quartz is used as a gas reaction passage; the metal spiral coil is used, so that corresponding other plasma generating imbalance can complete a chemical reaction through the synergic effect driving with the catalyst in the passage, so that the subsequent ingredient analysis of gas after a reaction is convenient; further, the application in aspects of the chemical engineering synthesis, greenhouse effect gas recovery utilization and renewable energy sources effective storage is realized; the advantages of high reaction selectivity, high energy utilization efficiency and the like are realized.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
Method for synthesizing phthaloyl amlodipine
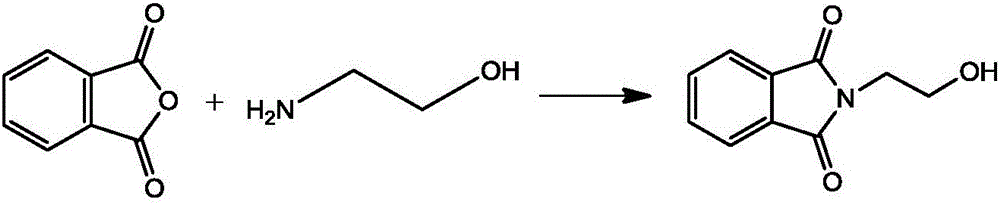
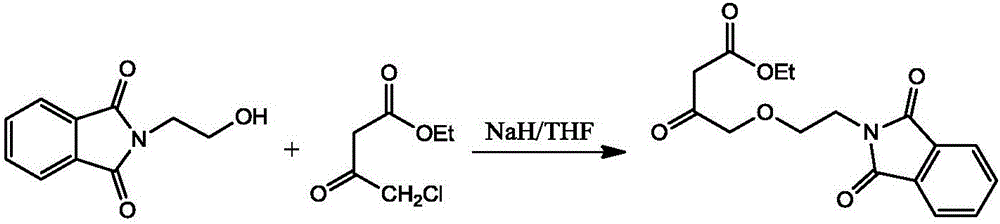
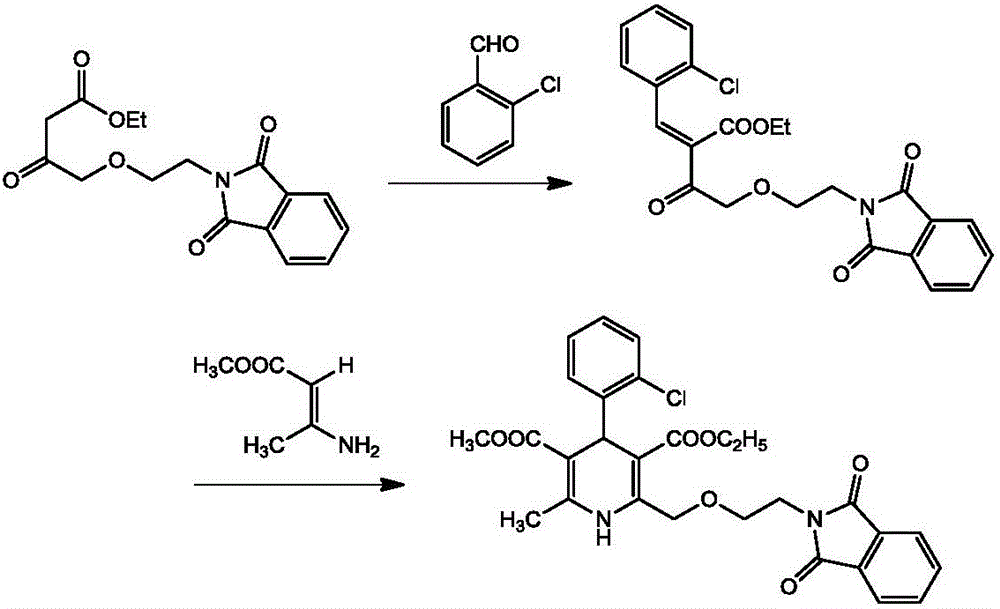
The invention relates to the field of drug synthesis, particularly to a method for synthesizing phthaloyl amlodipine. The method for synthesizing the phthaloyl amlodipine comprises the following steps: (1) synthesis of N-hydroxyethyl phthalimide; (2) synthesis of ethyl-4-(2-phthaloyl ethoxy) ethyl acetoacetate, wherein the ethyl-4-(2-phthaloyl ethoxy) ethyl acetoacetate is prepared by enabling the N-hydroxyethyl phthalimide to react with 4-chloroacetoacetate under a certain condition; (3) synthesis of phthaloyl amlodipine, wherein a target product is obtained by adding 2-chlorobenzaldehyde, acetic acid, piperidine and beta-methyl aminocrotonate to the ethyl-4-(2-phthaloyl ethoxy) ethyl acetoacetate in sequence under a certain condition to react with the ethyl-4-(2-phthaloyl ethoxy) ethyl acetoacetate, adding acetic acid to a reaction product after the reaction, and crystallizing, filtering, washing and drying the reaction product and the acetic acid. The method for synthesizing the phthaloyl amlodipine, provided by the invention, is efficient and easy to operate and can prepare the phthaloyl amlodipine with high yield and excellent quality.
Owner:千辉药业(安徽)有限责任公司
Method for extracting magnolol and honokiol from magnolia officinalis
ActiveCN101759532AResolve separabilityResolution timeOrganic chemistryOrganic compound preparationChemical reactionHonokiol
The invention discloses a method for extracting magnolol and honokiol from magnolia officinalis; the method includes that: magnolia officinalis powder and solid phase weak base reagent are mixed according to the weight ratio of 1:0.01-1, the mixture is grinded to obtain powder with granularity analysis result D90 of 10-200 um, and then water is added to the powder, the mixture is stirred sufficiently and is centrifuged to obtain sediment A and supernate A, acid X with pH value of 1.0-6.0 is added to the supernate A, then the supernate is centrifuged after standing to obtain sediment B as magnolol extractive. The invention firstly adopts mechanical chemical reaction technique to extract magnolol, and then adopts alkaline process to extract honokiol, has the advantages of high extraction rate, good separation effect of magnolol and honokiol, and short production cycle, and is the extraction and separation process with good popularization and application prospect.
Owner:ZHEJIANG UNIV OF TECH
Carbonyl starch oxidized by Fenton similar system, and preparation method of carbonyl starch
InactiveCN103509129AStrong oxidation abilityOvercome the problem of poor selectivity of the preparation processWater bathsNitrate salts
The invention discloses a carbonyl starch oxidized by a Fenton similar system, and a preparation method of the carbonyl starch. The Fenton similar system is composed of a cerous nitrate salt-a catalyst-H2O2; the catalyst is ferrous sulfate or cuprous sulfateb; starch is oxidized in aqueous phase at a temperature of 20 to 60 DEG C. The preparation method comprises following steps: (1) 30 to 50 weight portions of starch and 60 to 170 weight portions of water are mixed so as to obtain a turbid liquid, and the turbid liquid is subjected to gelatinization in water bath for 0.5 to 2h at a temperature of 50 to 70 DEG C; (2) 0.3 to 2.0 weight portions of ceric ammonium nitrate and 0.0015 to 0.0075 weight portion of the catalyst are added and mixed fully, pH value of the reaction solution is adjusted to 2.5 to 4.0, and the reaction solution is reacted for 10 to 24h at a temperature of 20 to 45 DEG C in enclosed conditions; (3) 1.5 to 10 weight portions of H2O2 is added, the temperature is adjusted to 45 to 60 DEG C, and the mixture is reacted for 3 to 7h; and (4) after that, the mixture is subjected to suction filtration, is washed for 3 times with acetone, and is dried in a vacuum oven for 12 to 24h at a temperature of 40 to 60 DEG C so as to obtain the carbonyl starch containing 10 to 20% of carbonyl and 2 to 6% of carboxyl. According to the preparation method, both carbonyl and carboxyl are introduced into starch at the same time, so that functional groups of starch molecules are increased, and application area of oxidized starch is widened.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
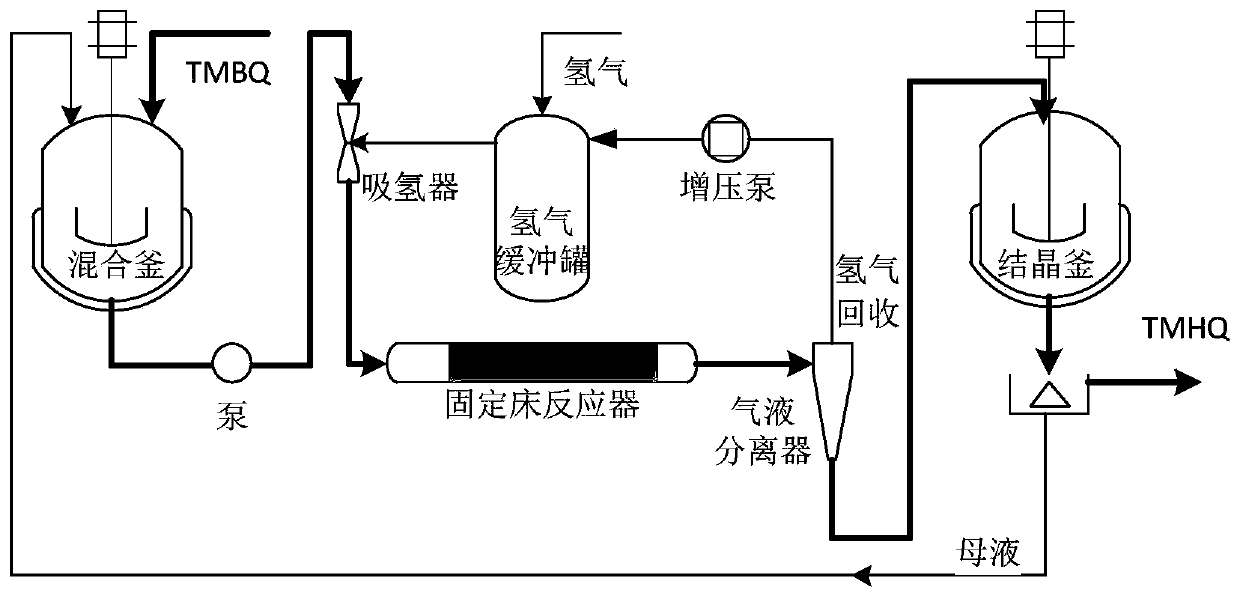
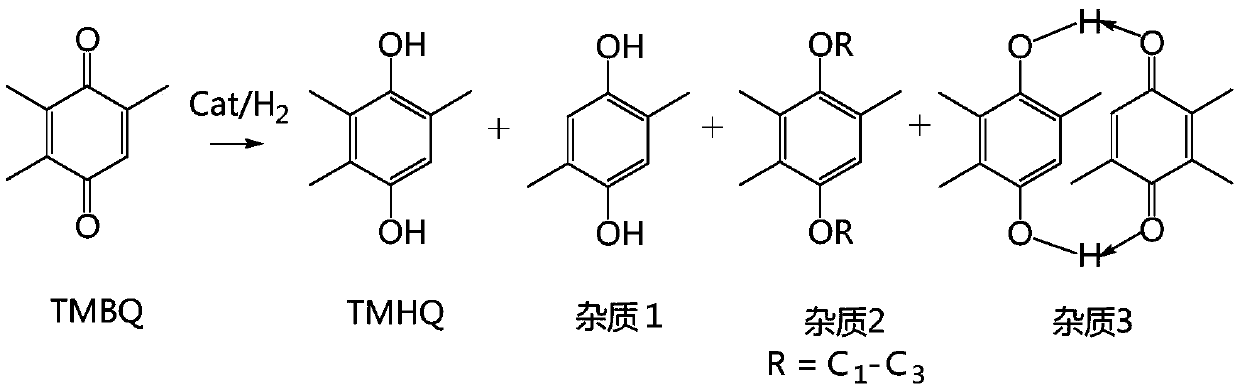
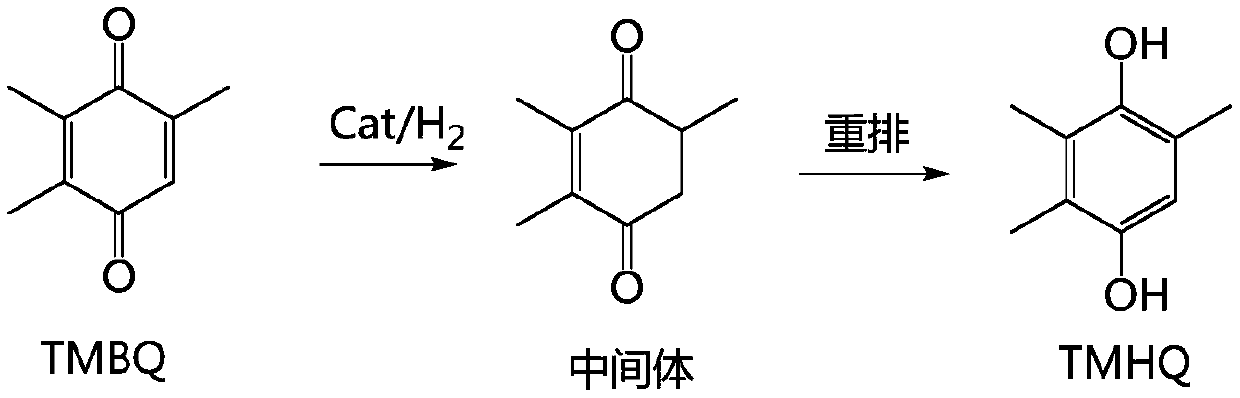
Synthesis method and device of 2,3,5-trimethylhydroquinone
InactiveCN111253218AHigh amount of hydrogenControl contentLiquid degasificationOrganic chemistryAlkanePtru catalyst
The invention discloses a synthesis method and device of 2,3,5-trimethylhydroquinone. According to the synthesis method for 2,3,5-trimethylhydroquinone, 2,3,5-trimethylbenzoquinone (TMBQ) and an alcohol-aromatic hydrocarbon or alcohol-alkane mixed solvent system are mixed, then a reaction solution is fully mixed with hydrogen through a hydrogen absorber, then the mixture enters a fixed bed filledwith a noble metal catalyst to complete a hydrogenation reaction, and 2,3,5-trimethylhydroquinone (TMHQ) is obtained. According to the technical scheme provided by the invention, the reaction selectivity is improved, the side reaction is effectively inhibited, the impurity content of the product is reduced, the purity of 2,3,5-trimethylhydroquinone (TMHQ) is improved, the production process is simplified, the emission of three wastes is reduced, and the method has good environmental protection benefits.
Owner:SHANGYU NHU BIOCHEM IND +2
Electrode and preparation method thereof
InactiveCN105274557AImprove conductivityLower resistanceElectrode shape/formsElectricityConductive materials
The invention relates to an electrode used in the electrolytic industry and a preparation method thereof. The electrode comprises a conductive rod and a substrate, wherein the substrate is composed of a three-dimensional network metal skeleton and a conductive material. The three-dimensional network metal skeleton is made of metallic mesh plates in a welded mode, the conductive rod is embedded in the substrate, and electro-catalytic active layers cover the surface of the substrate. According to the electrode, the electric conductivity is high, the service life is long, the reaction selectivity of the electrode is good, the electrode is not prone to passivity, the production cost is low and the application value is good.
Owner:XIAMEN UNIV OF TECH
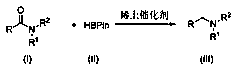
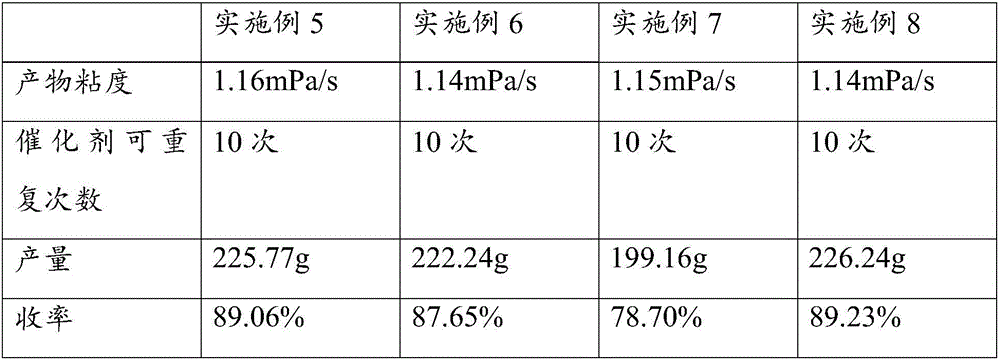
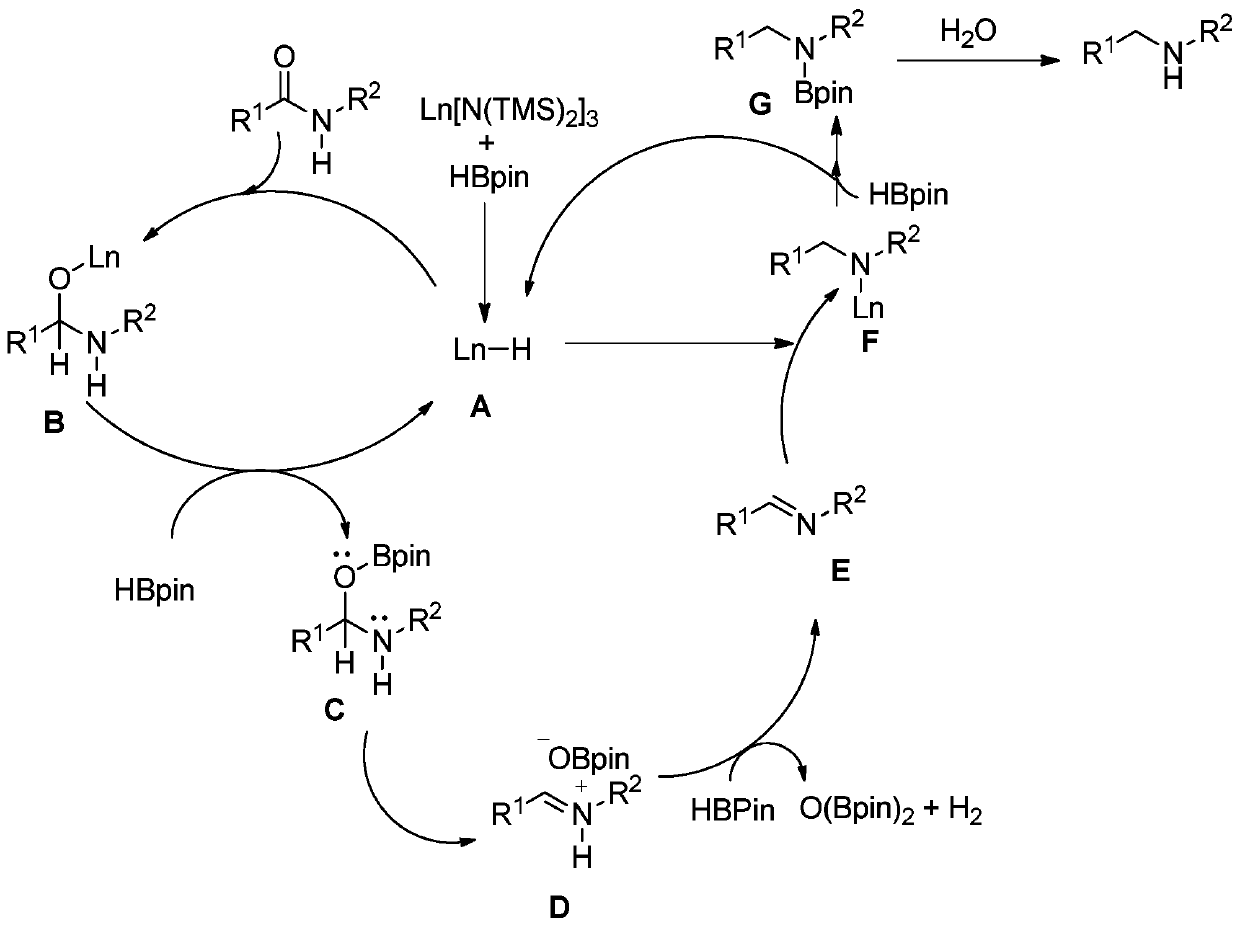
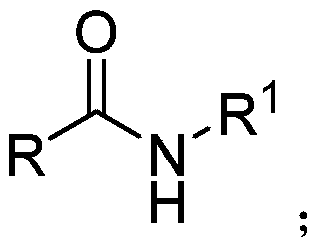
Method for synthesizing tertiary amine derivative by catalyzing hydroboration reaction of tertiary amide by rare earth
ActiveCN110803995AImprove qualityProcess stabilityOrganic compound preparationChemical recyclingPtru catalystBiochemical engineering
The invention discloses a method for synthesizing a tertiary amine derivative by catalyzing a hydroboration reaction of tertiary amide by rare earth. Under the protection of nitrogen, in a rare earthcatalysis system, the tertiary amine derivative is prepared through a hydrogen transfer reduction reaction catalyzed by rare earth with tertiary amide and pinacol borane as raw materials. The method has the advantages and effects that a novel synthesis process is utilized, and the tertiary amine derivative prepared by using the method disclosed by the invention is high in quality and stable in process; the raw materials are widely sourced or easy to prepare and store, and the reaction yield is high; and in addition, a catalyst can be directly commercially purchased or is easily, directly and simply synthesized, and can be used in actual production.
Owner:WENZHOU MEDICAL UNIV
Method for synthesizing high-yield nandrolone
The invention relates to a method for synthesizing high-yield nandrolone. The method comprises the following synthesizing steps of: (1) etherification reaction: absolute ethyl alcohol and triethyl orthoformate are put into a reactor which is provided with an agitation device to be agitated, 19-norandrostendione, and pyridine hydrobromate and triethylamine are then put into the reactor and are agitated, freeze crystallized, sprinkled and centrifugally filtered to obtain wet etherate; (2) reduction hydrolysis reaction: in a reaction vessel, methanol is put at first to be mixed, the etherate and sodium borohydride are then put, acid is added to regulate pH, vacuum concentration is carried out, water is flushed, centrifugation is carried out to obtain a reduction hydrolysate, i.e. crude nandrolone, and the crude nandrolone is dried; and (3) crude product refining: the crude nandrolone, ethyl acetate and petroleum ether are put into a tank at one time and are heated to dissolve, cooled, centrifuged and dried, so that refined nandrolone is obtained. The method adopts a new catalyst-pyridine hydrobromate, so that a hydrolysis phenomenon cannot be caused after protection materials are added, and thus, the protection yield and quality are improved.
Owner:YICHENG GOTO PHARMA
High-yield Boldenone synthesis method
The invention relates to a high-yield Boldenone synthesis method. The method comprises the following steps of: 1, etherification reaction: adding absolute ethyl alcohol and triethyl orthoformate in a reaction kettle, stirring, adding 1.4-ADD, pyridine hydrobromide and triethylamine, freezing for crystallization, washing with absolute ethyl alcohol, and filtering in a swinging manner to get the wet etherate; 2, reduction and hydrolysis reaction: firstly, adding methyl alcohol in the reaction kettle, stirring, then, adding etherate pyridine and sodium borohydride, preserving heat for reaction, and carrying out thin layer chromatography, after the reduction reaction, regulating the temperature, regulating pH, concentrating the mixture to be in dense slurry form, adding water, cooling, centrifuging to get reduced hydrolysate, namely a Boldenone crude product, and drying; and 3, refining of crude product: adding the Boldenone crude product, methyl alcohol and water in the production kettle, heating with steam, cooling, and centrifuging. In the method, a new catalyst, namely pyridine hydrobromide, is used, thus, the yield and the quality of the product are improved. The method realizes one-step preparation of Boldenone, and in comparison with the DDQ (dihydro dicyan benzoquinone) dehydrogenation used in the past, the cost of products is saved in the synthesis method.
Owner:YICHENG GOTO PHARMA
Low molecular siloxane and preparation method thereof as well as used catalyst and preparation method thereof
ActiveCN106317097ANarrow molecular weight distributionGood catalyticSilicon organic compoundsHeterogenous catalyst chemical elementsPlatinumHydrogen
The invention provides a preparation method of low molecular siloxane as well as a used catalyst and a preparation method thereof. The catalyst is a solid acid catalyst formed by a supporter loaded active component, wherein the active component at least contains platinum and zirconium oxide in a ratio of 1:(1-5). The preparation method of the low molecular siloxane comprises the following steps: by taking high hydrogen-containing silicone oil as a raw material, carrying out telomerization at the temperature of 50-110 DEG C in presence of a blocking agent and the catalyst, so that the low molecular siloxane is obtained. A novel catalyst is provided for preparation of the low molecular siloxane by adopting a telomerization process, and the catalyst has the advantages of high catalysis efficiency, specific product and high reutilization frequency.
Owner:岳阳凯门水性助剂有限公司
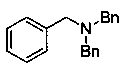
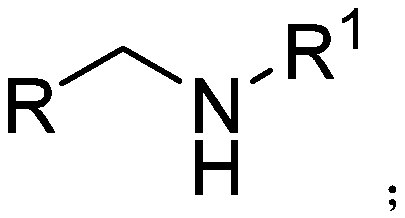
Secondary amine derivative synthesized through rare earth catalysis, and preparation method thereof
ActiveCN110818576AEasy to getWide variety of sourcesAmino preparation from aminesOrganic compound preparationPtru catalystReaction temperature
The invention discloses a secondary amine derivative synthesized through rare earth catalysis, and a preparation method thereof. According to the preparation method, the secondary amine derivative isprepared by carrying out a reaction on reactants of secondary amide and pinacol borane; a rare earth catalyst bis(trimethylsilyl) amino yttrium is added; the reaction temperature is 100-140 DEG C, andthe reaction time is 20-25 h; the whole reaction is carried out under a normal pressure, and the reaction conditions are mild, easy to achieve and safe; the method is simple and convenient to operateand high in reaction selectivity, can directly synthesize the target product without intermediate product separation, can obtain the target product only through a reaction under a normal pressure, issimple in reaction process, has the yield of 90% at most, substantially simplifies the process engineering, reduces the energy consumption, and has high yield; the reaction raw materials are stable and easy to store; a series of secondary amine derivatives can be prepared; and the method has high substrate universality so as to provide the good guarantee for development of related substances related to secondary amine derivatives, and is suitable for large-scale application and popularization.
Owner:WENZHOU UNIVERSITY
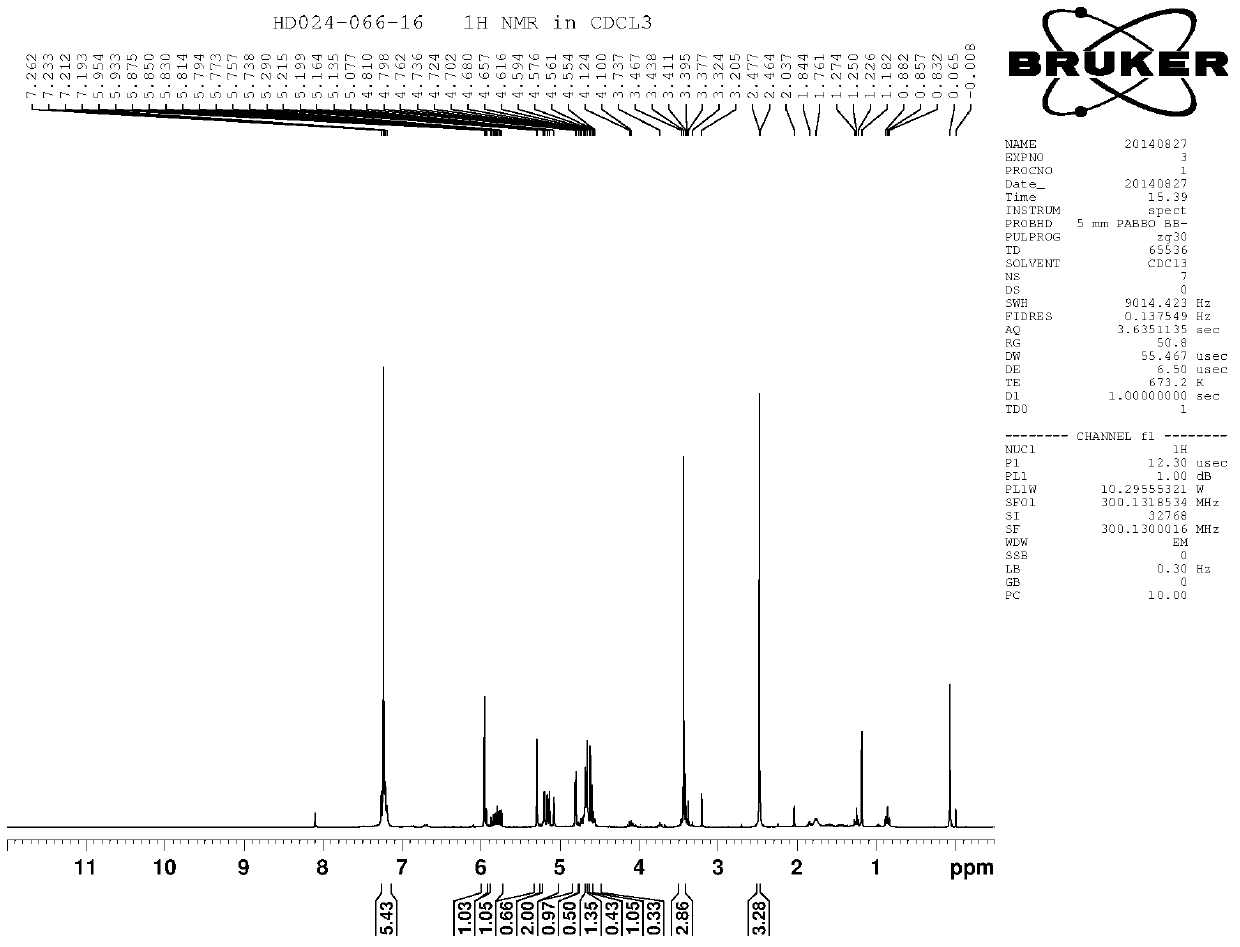
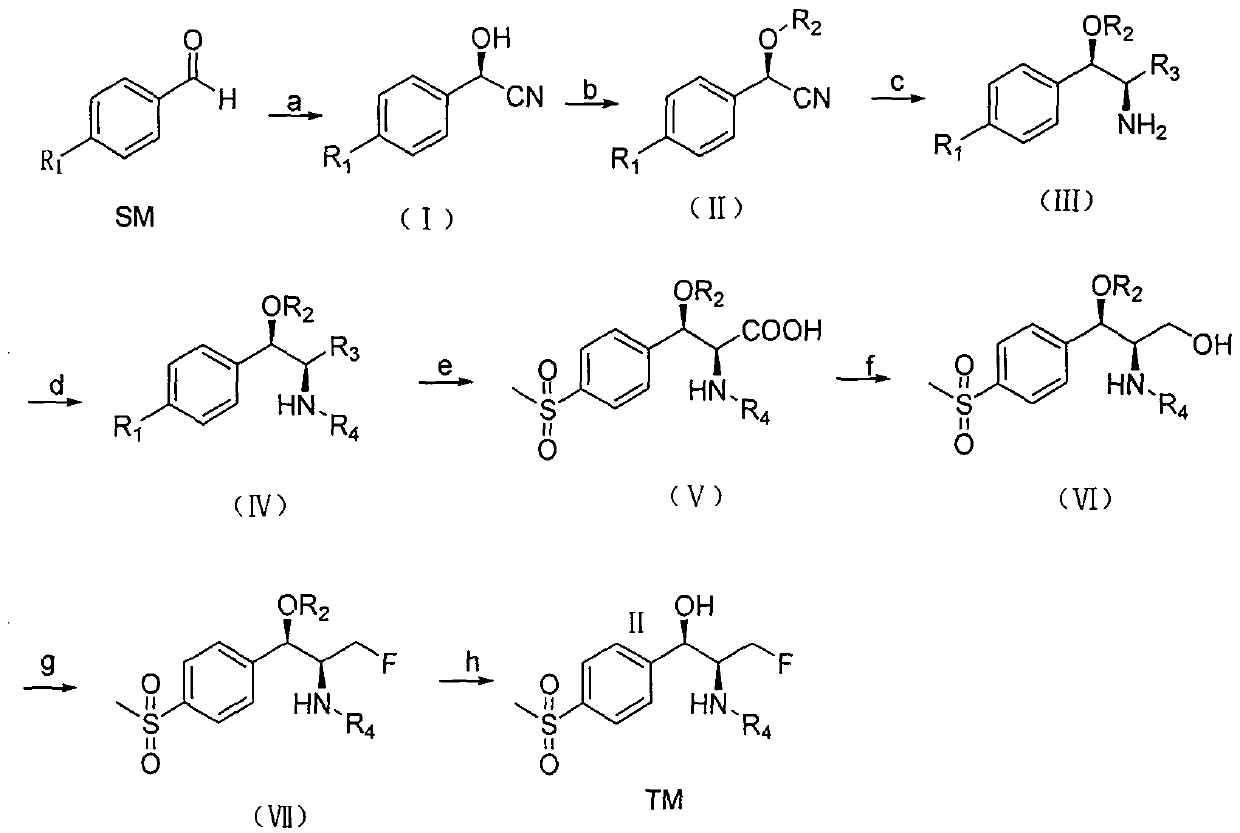
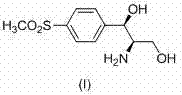
A kind of Florfenicol intermediate, its preparation method and the preparation method of Florfenicol
ActiveCN106187837BSimple chemical reactionLow costGroup 4/14 element organic compoundsOrganic compound preparationMethiodideTert-Butyloxycarbonyl protecting group
The invention discloses a florfenicol midbody, a preparation method thereof and a preparation method of florfenicol, and belongs to the field of veterinary drug preparation. The invention provides the florfenicol midbody shown in a formula IV, wherein R1 is dimethyl sulfide or methylene sulphonyl or methyl sulphonyl, R2 is TBS- or TMS- or MOM- or THP-, R3 is shown in the description, and R4 is dichloroacetyl or benzoyl or t-butyloxycarboryl. The invention further provides a preparation of the florfenicol midbody shown in the formula IV and a preparation method of florfenicol. The obtained florfenicol is low in cost, simple in technology and high in yield, and the product chirality purity reaches up to 98%.
Owner:HEADING NANJING PHARMTECH CO LTD
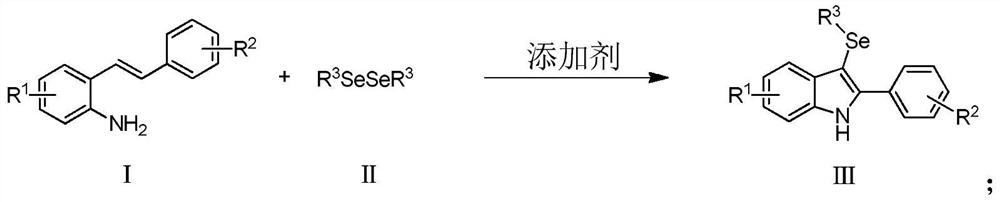
Method for synthesizing 3-selenoindole derivative
ActiveCN111848488AEfficient synthesisImprove universalityOrganic chemistryOrganic synthesisCombinatorial chemistry
The invention belongs to the technical field of organic synthesis, and particularly relates to a method for synthesizing a 3-selenoindole derivative. Under the action of a non-metal additive, a compound 2-styryl aniline derivative and organic diselenide are used as raw materials, and tandem cyclization reaction is realized through a 'one-pot method', so that the 3-selenoindole derivative with a diversified structure is synthesized. The method is easy and convenient to operate, high in reaction selectivity, wide in universality, environmentally friendly and capable of avoiding use of metal reagents.
Owner:WENZHOU MEDICAL UNIV
Graphene oxide modified by betaine type zwitterionic compound and preparation method thereof
The invention discloses graphene oxide modified by a betaine type zwitterionic compound and a preparation method thereof. The preparation method is as follows: firstly, the surface of graphene oxide is subjected to mercapto-treatment by hydrolysis of a mercapto-containing silane coupling agent; then, ethylene-based carboxylic acid or sulfonate betaine-type The zwitterionic compound is grafted onto the surface of the graphene oxide to obtain the graphene oxide modified by the betaine type zwitterionic compound. The betaine-type zwitterionic compound-modified graphene oxide prepared by the present invention not only has excellent biocompatibility, but also has a simple and easy preparation method, high reaction efficiency and selectivity, easy regulation, and simple post-treatment of the product. convenient. This approach provides a new avenue to improve the biocompatibility of graphene oxide. The graphene oxide modified by the betaine-type zwitterionic compound will have broad application prospects in the fields of biomedical materials such as medical devices, tissue engineering scaffolds, drug sustained release, and anti-bacterial adhesion.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of citral
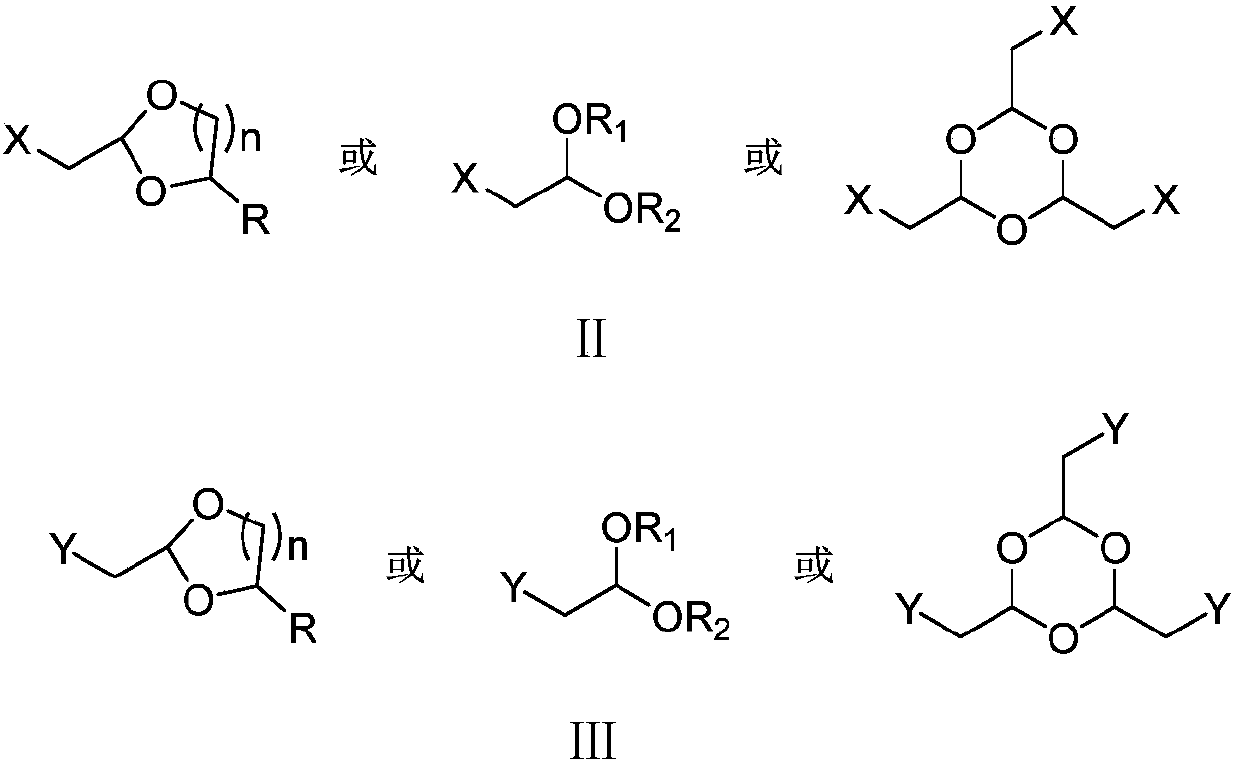
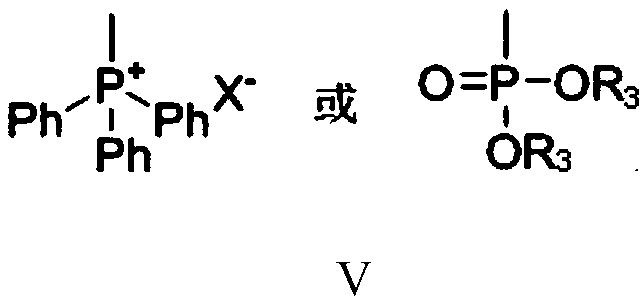
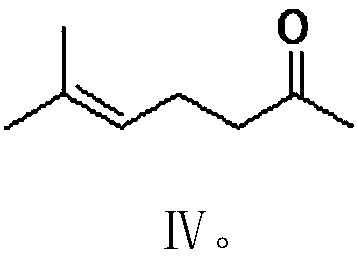
PendingCN111574349AImprove economyHigh selectivityOrganic compound preparationOrganic chemistry methodsPhosphorous acidGrignard reagent
The invention provides a preparation method of citral. The method comprises the following steps: carrying out a Grignard reaction or substitution reaction on halogenated acetaldehyde acetal or halogenated paraldehyde and a reagent 1 (magnesium powder, triphenylphosphine or triphosphite) to obtain a corresponding Grignard reagent or Wittig reagent, reacting the obtained Grignard reagent or Wittig reagent with 6-methyl-5-hepten-2-one, and successively conducting acidifying and deprotection to obtain citral. The preparation method has the advantages of usage of cheap and accessible raw materials,simple operation steps, high reaction atom economy, high selectivity, high yield, high purity, low product cost, and suitability for green industrial production.
Owner:XINFA PHARMA
Synthetic process method of high-density hydrocarbon
ActiveCN105542852AHigh yieldIncrease productivityLiquid carbonaceous fuelsTreatment with hydrotreatment processesIsomerizationReaction temperature
The invention discloses a synthetic process method of high-density hydrocarbon. The synthetic process method comprises the following steps of the first step, hydrogenation of a raw material, industrial grade dicyclopentadiene raw material of which the mass percent is 85 percent is adopted for a hydrogenation reaction, meanwhile a separate-out distillation method is adopted to refine endo-tetrahydrodicyclopentadiene; the hydrogenation reaction is performed on the raw material on a bed layer filled with a metal catalyst; the hydrogen-oil ratio is (300 to 1000):1; the space velocity is 5-25h<-1>; the inlet pressure of a hydrogen reactor is 1.2 to 2.2 MPa; the inlet temperature of the reactor is 100 to 140 DEG C; the second step, a space isomerization reaction is adopted to generate an exo-tetrahydrodicyclopentadiene product; a molecule space isomerization reaction of an intermediate product is catalyzed by chloro metal; an inert solvent is adopted as an isomerization solvent; the reaction temperature is 70 to 150 DEG C; the reaction time is 1 to 3h; distillation separation is performed on the inert solvent under the condition of 80 to 85 DEG C to refine the high-density hydrocarbon. Compared with other processes, the liquid yield of the exo-tetrahydrodicyclopentadiene prepared by the synthetic process method is about 99 percent, the space velocity is higher, i.e. the charging rate is higher, and the production efficiency is higher.
Owner:ZHUHAI CHANGLIAN PETROCHEM EQUIP
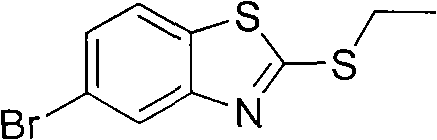
Preparation method of 2-methylthio-5-benzothiazole
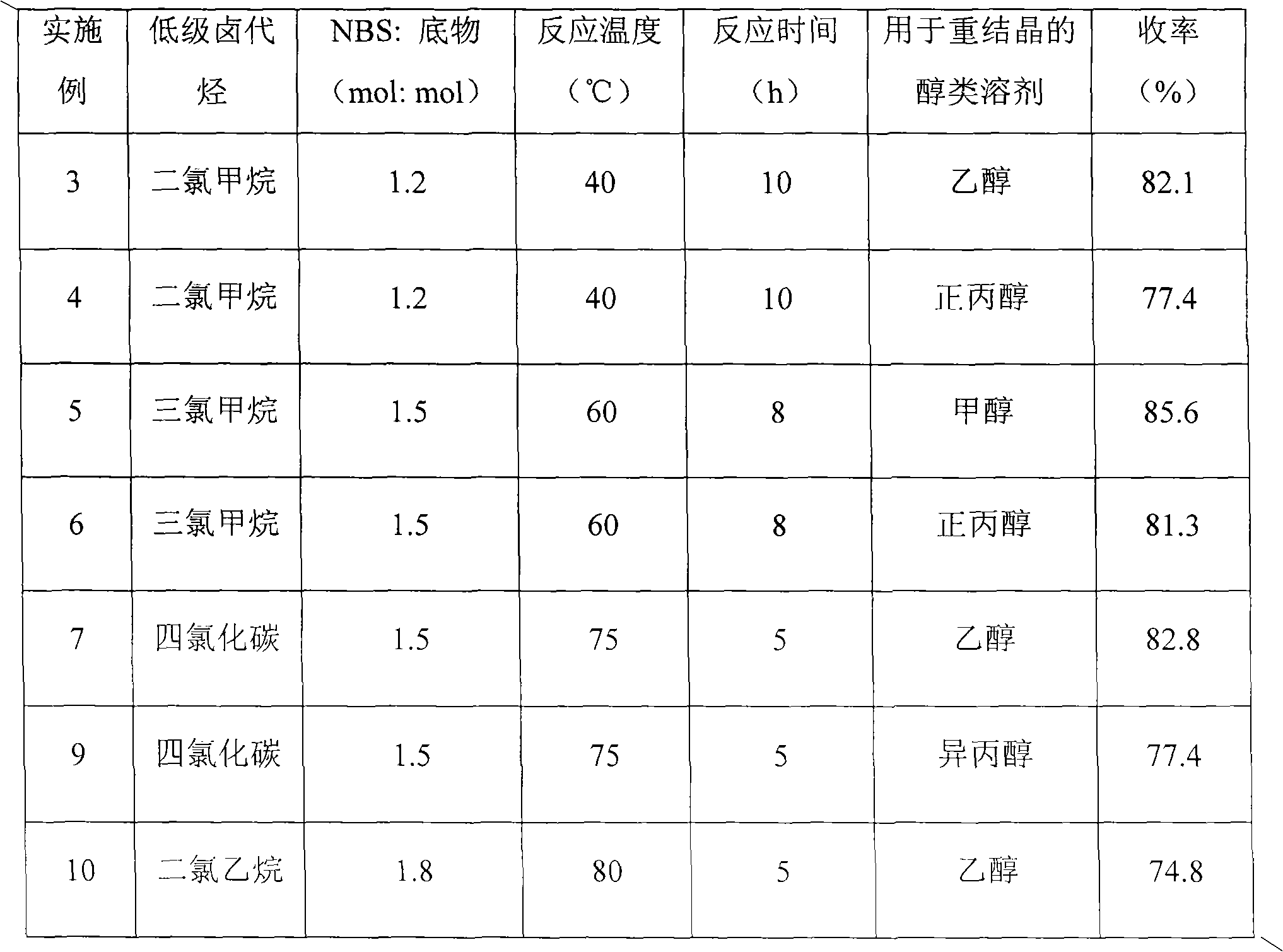
InactiveCN102010385AStrong reaction selectivityHigh reaction yieldOrganic chemistryHalohydrocarbonDistillation
The invention discloses a preparation method of 2-methylthio-5-benzothiazole, successively comprising the following steps: 1) mixing ethanol, a sodium hydroxide solution, 2-mercaptobenzothiazole and bromoethane to react until the 2-mercaptobenzothiazole is completely reacted; 2) cooling the product obtained by step 1) to room temperature, splitting phases, washing, and carrying out reduced pressure distillation and the like to obtain 2-methylthio benzothiazole; 3) dissolving the 2-methylthio benzothiazole into low-grade halohydrocarbon, and adding N-bromosuccinimide to react; and 4) after the reactive fluid obtained in step 3) is cooled to room temperature, successively filtering, washing, drying and carrying out reduced pressure distillation to obtain the wax-state solid product of the salmon 2-methylthio-5-benzothiazole. The 2-methylthio-5-benzothiazole prepared according to the method disclosed in the invention has the characteristics of simple technology, high yield, low cost, small three-waste discharge and the like.
Owner:ZHEJIANG UNIV
Preparation method for Co-Ni dual-metal-loaded TiO2(B) photocatalysis material and application of photocatalysis material
InactiveCN108855097ALow costStable in natureHeterogenous catalyst chemical elementsHydrogen productionEmulsionHydrogen
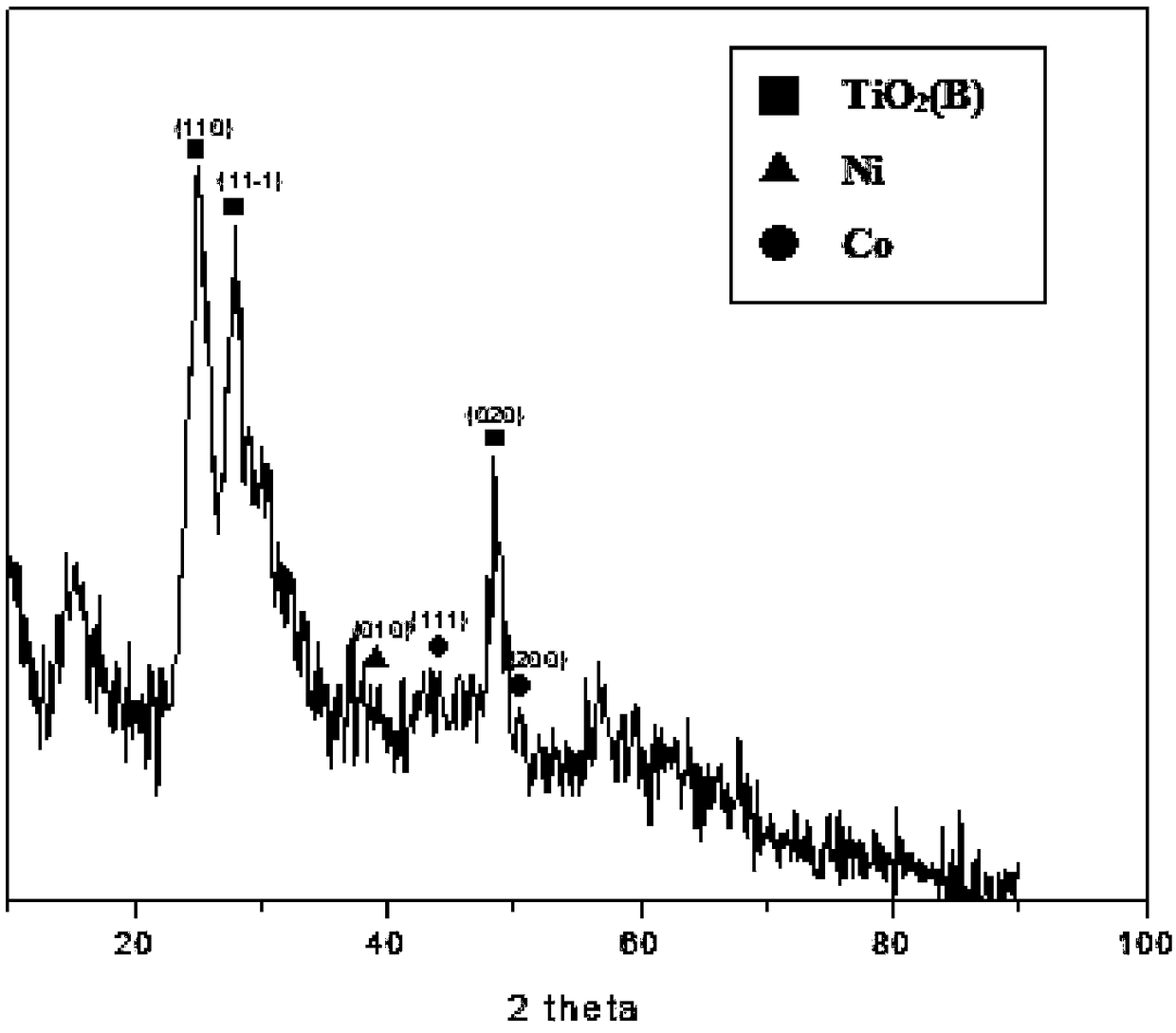
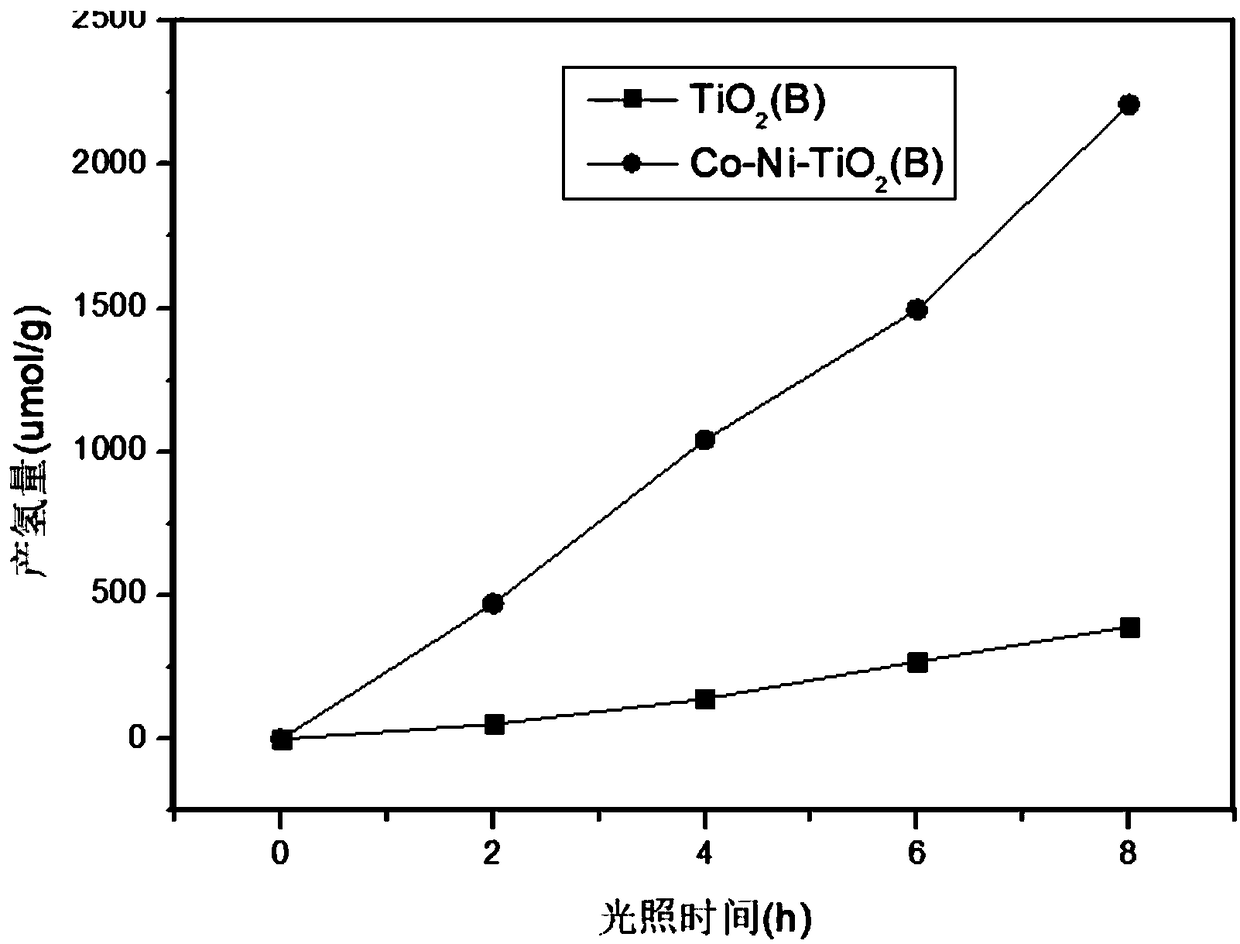
The invention discloses a preparation method for a Co-Ni dual-metal-loaded TiO2(B) photocatalysis material and application of the photocatalysis material in generating hydrogen in a water photohydrolysis system. The method mainly comprises the following steps: weighing TiO2(B) and Ni(NH3)6Cl2, [Co(NH3)5Cl]Cl2 into a beaker, and adding deionized water into the beaker, and performing ultrasonic treatment for enabling TiO2 (B) and ammonate to uniformly mix; regulating the pH value to 10.8-11.2 by ammonia water and hydrochloric acid; shaking obtained emulsion for 0.5-2 hours to obtain TiO2(B) emulsion adsorbed with Ni(NH3)6Cl2 and [Co(NH3)5Cl]Cl2 on surface; performing light illumination for 0.5-1.5 hours, reducing the ammonate, alternatively flushing a sample obtained after reaction with deionized water and liquor, and drying in a vacuum drying oven to obtain the Co-Ni dual-metal-loaded TiO2(B) photocatalysis material. Compared with the original TiO2(B), the Co-Ni dual-metal-loaded TiO2(B) photocatalysis material has more active sites, a lower current carrier recombination rate and higher catalytic activity. Moreover, the method is low in implementation cost, is convenient, simple andeasy to implement; and the obtained material is stable in structure, is lasting in activity, and is an efficient economical method.
Owner:TIANJIN UNIV
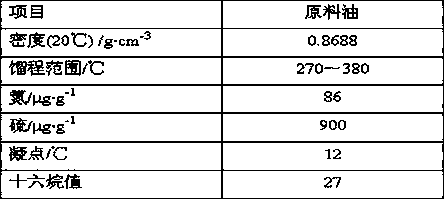
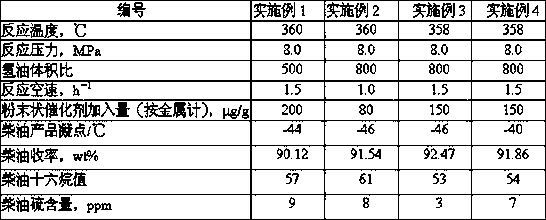
A hydrogenation method for increasing the cetane number of inferior diesel oil
ActiveCN106675648BRealize comprehensive utilizationQuality assuranceTreatment with hydrotreatment processesHydrocarbon oils treatment productsHydrogenation reactionSulfur
The invention discloses a hydrogenation method for enhancing cetane number of inferior diesel. The method comprises the following steps: (1) sufficiently mixing the inferior diesel raw material and a powdery hydrofining catalyst in a mixer to obtain a catalyst-raw oil feed mixture; (2) adding the feed mixture and hydrogen into an ebullated bed reactor from the reactor bottom filled with a mixture of a hydrodefreezing catalyst and a hydrogenation modification catalyst, and carrying out hydrogenation reaction; (3) discharging the reaction stream containing the powdery hydrofining catalyst out of the top of the ebullated bed reactor into a stable reactor, and carrying out supplementary hydrofining; and (4) carrying out solid-liquid separation on the material obtained in the step (3), and sending the liquid phase into a fractionating system to obtain the high-quality low-pour-point low-sulfur high-cetane-number diesel product. The method reasonably combines the temperature drop in the hydrodefreezing process and the temperature rise in the hydrogenation modification process, thereby lowering the low-pour-point high-cetane-number diesel, lowering the hot-spot temperature of the device and prolonging the operating cycle on the premise of ensuring the high diesel yield.
Owner:CHINA PETROLEUM & CHEM CORP +1
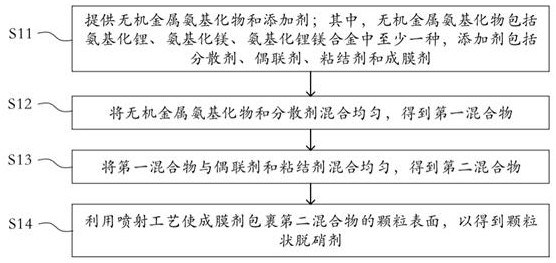
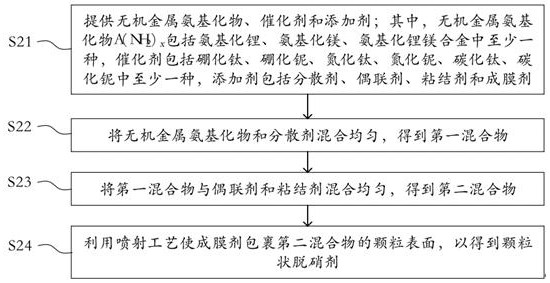
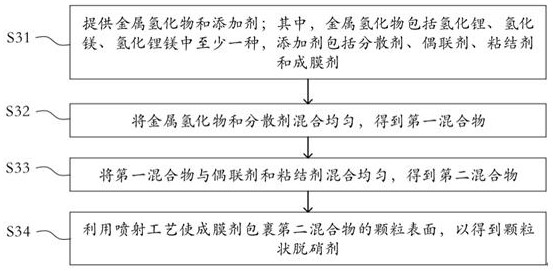
Denitration agent, preparation method thereof and flue gas purification method
ActiveCN114405241AHigh activityStrong reaction selectivityGas treatmentDispersed particle separationMg alloysFlue gas
The invention discloses a denitration agent, a preparation method thereof and a flue gas purification method, and belongs to the technical field of flue gas denitration. The denitration agent comprises an inorganic metal amide and an additive, or comprises a metal hydride and an additive, the inorganic metal amide comprises at least one of lithium amide, magnesium amide and a lithium amide magnesium alloy, and the metal hydride comprises at least one of lithium hydride, magnesium hydride and lithium magnesium hydride. Compared with an organic nitrogen-containing compound adopted in the prior art, the inorganic metal amide has high activity and strong selectivity, and the gas product is only ammonia gas NH3 and is directly used for reducing nitrogen oxide NOx in flue gas; the metal hydride gas product is only hydrogen H2 and also has a reduction effect on nitrogen oxides NOx. According to the application, the denitration efficiency can be improved, and the problem of ammonia escape in the flue gas denitration process can be relieved.
Owner:江苏海默环保科技有限公司
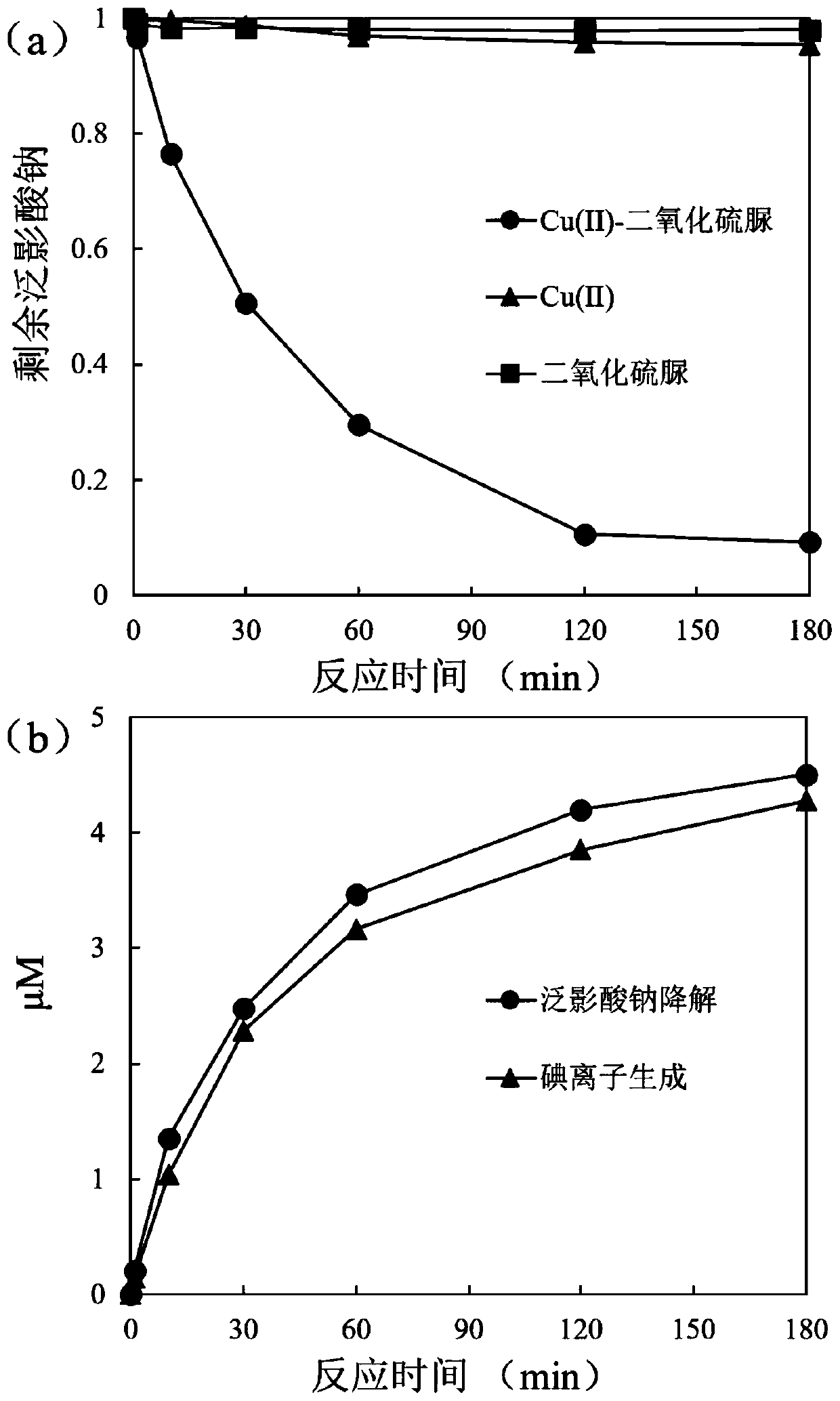
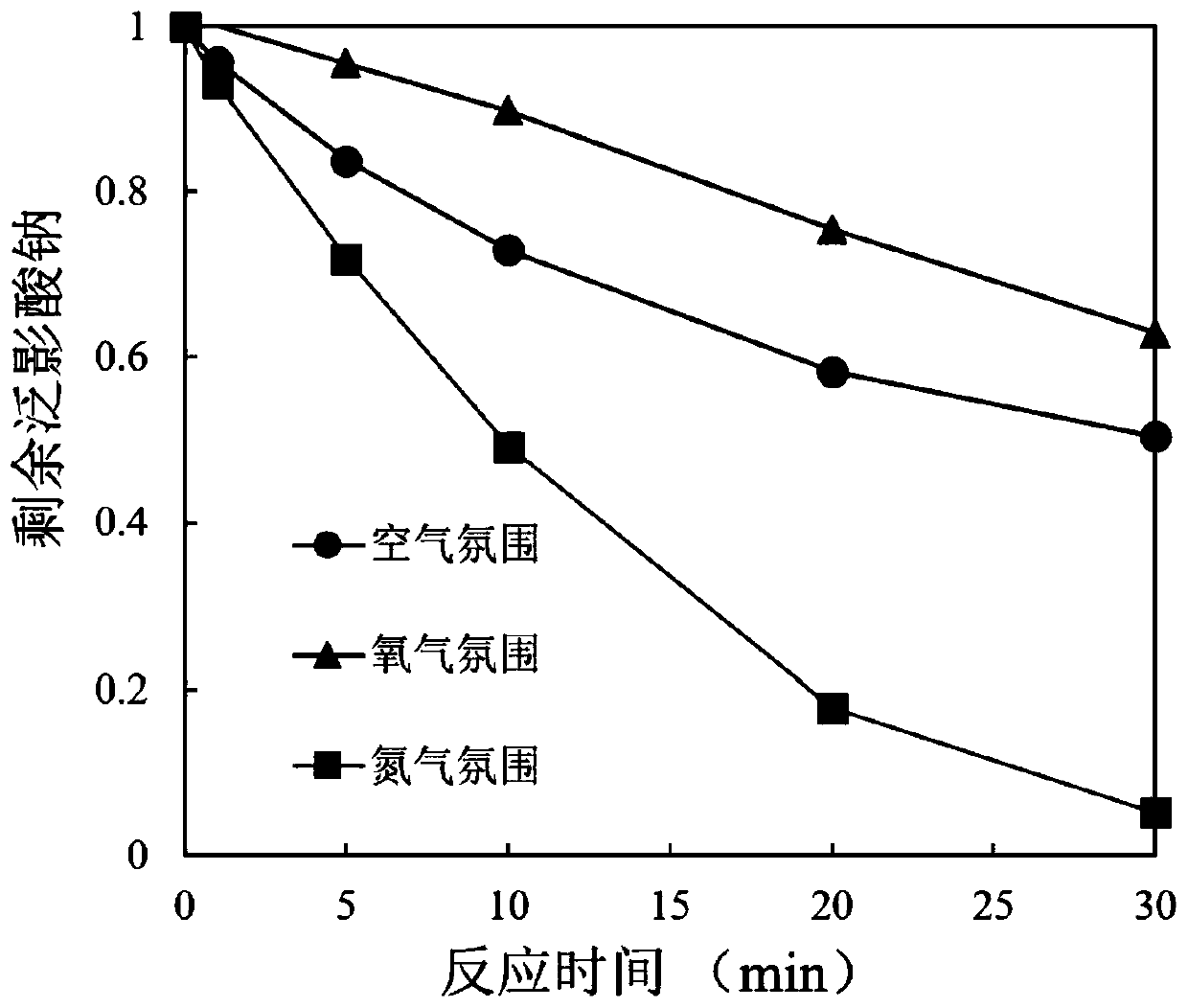
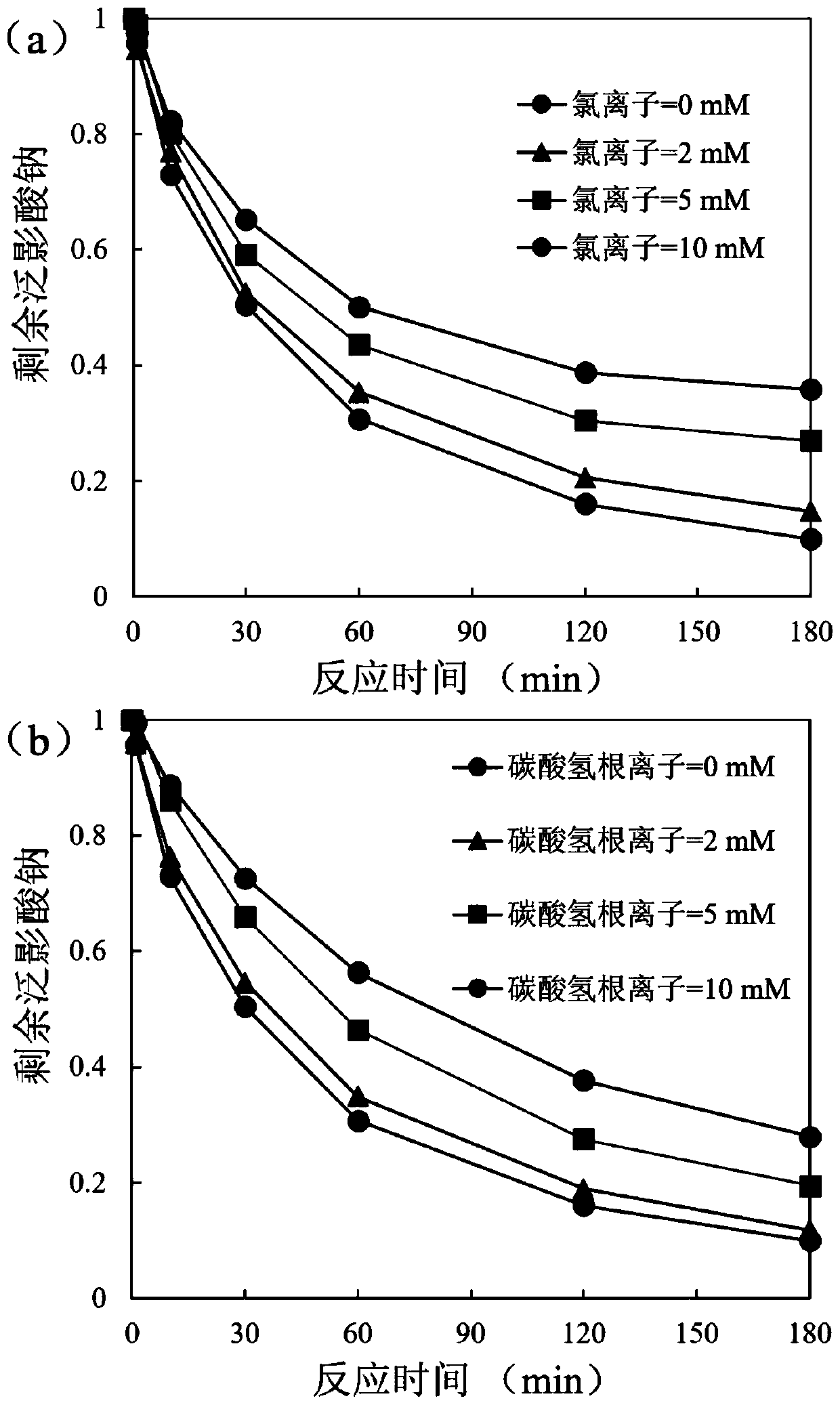
Water treatment method for reducing and degrading halogenated organic matters by using copper-activated thiourea dioxide
ActiveCN111285454AIncreased toxicityLow priceWater contaminantsWater/sewage treatment by reductionWaste materialReducing agent
The invention relates to a water treatment method for reducing and degrading halogenated organic matters by copper-activated thiourea dioxide, and belongs to the technical field of wastewater treatment. The method comprises the following steps: adjusting the pH value of the halogenated organic matter wastewater to 6-9, and adding copper ions until the concentration is 0.05-1 mg / L or adding copper-containing solid metal until the concentration is 1-100 mg / L; adding thiourea dioxide of which the concentration is 5-1000 times of the molar concentration of the pollutants, adding buffer salt or alkali liquor to maintain the pH value at 6-9, stirring and uniformly mixing, and reacting at room temperature for 2-10 hours. The added copper ions can be recycled, and no metal mud waste is generated;the reducing agent thiourea dioxide is low in price, and decomposition products are non-toxic and pollution-free; no energy input or complex reaction equipment is needed in the reaction process, the operation is simple and convenient, the reaction is efficient, the effect is stable, the reaction condition requirement is low, the interference of other coexisting inorganic ions and organic matters in the water on the action effect of the method is weak, the reaction selectivity is high, and the halogenated organic pollutants in the water can be removed in a targeted manner.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
A radio frequency plasma-catalyst synergistic reaction device
InactiveCN106975349BImprove energy efficiencyStrong reaction selectivityPhysical/chemical process catalystsDispersed particle separationChemical reactionHigh energy
The invention discloses a radio frequency plasma-catalyst synergistic reaction device, which includes a non-equilibrium plasma reaction chamber, a hollow tubular metal spiral coil with a cooling channel, a catalytic body with a catalyst, an air inlet closed end and an air inlet end. Sealing flange, exhaust closed end and exhaust end sealing flange, as well as electromagnetic shielding cover and other components. The invention is a reaction device designed for the synergistic effect of inductively coupled radio frequency plasma and catalysts. It uses a non-equilibrium plasma reaction chamber made of quartz as a gas reaction channel, and uses a metal spiral coil to generate corresponding non-equilibrium plasma in the channel with the catalyst. Synergy drives the completion of chemical reactions to facilitate subsequent analysis of the components of the reacted gases, thereby realizing applications in chemical synthesis, greenhouse gas recycling and efficient storage of renewable energy. It has the advantages of strong reaction selectivity and high energy utilization efficiency.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
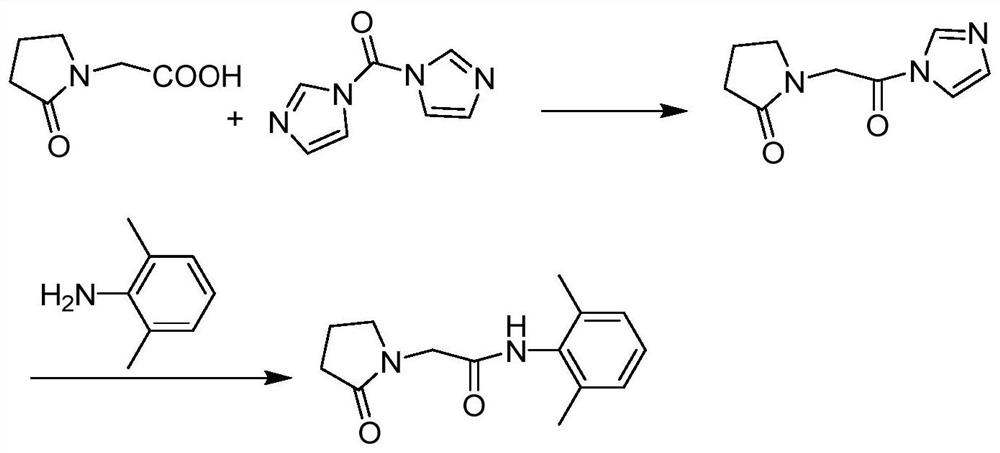
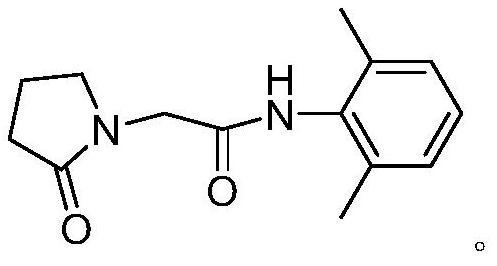
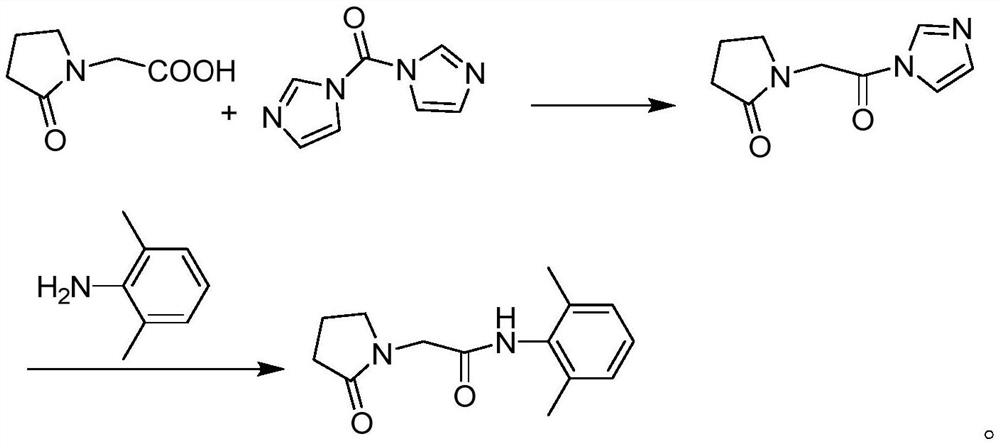
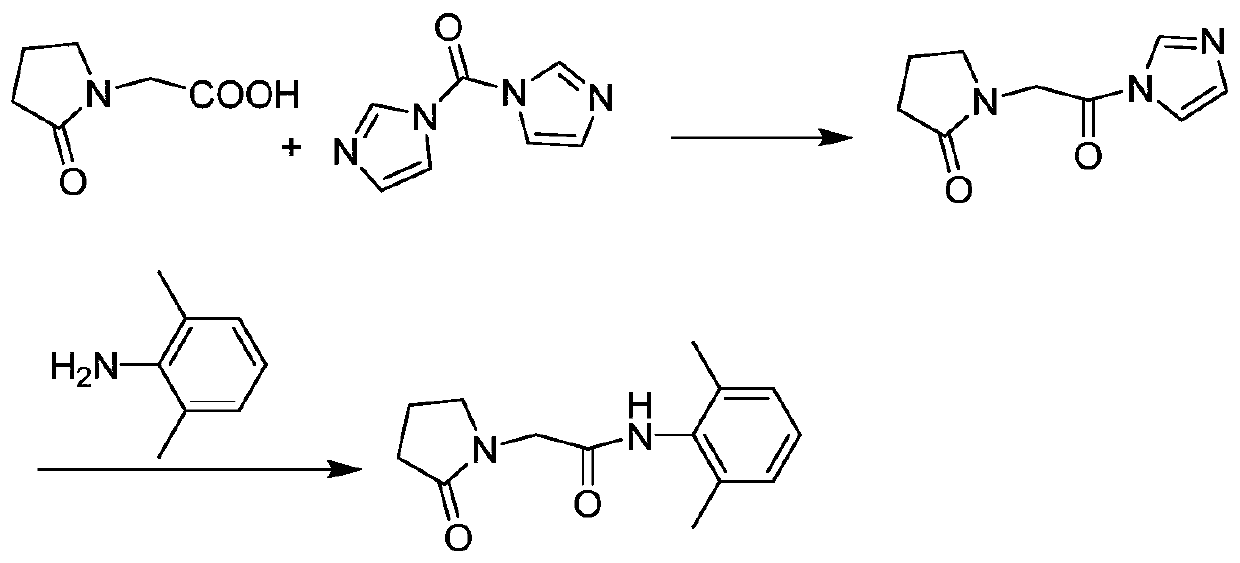
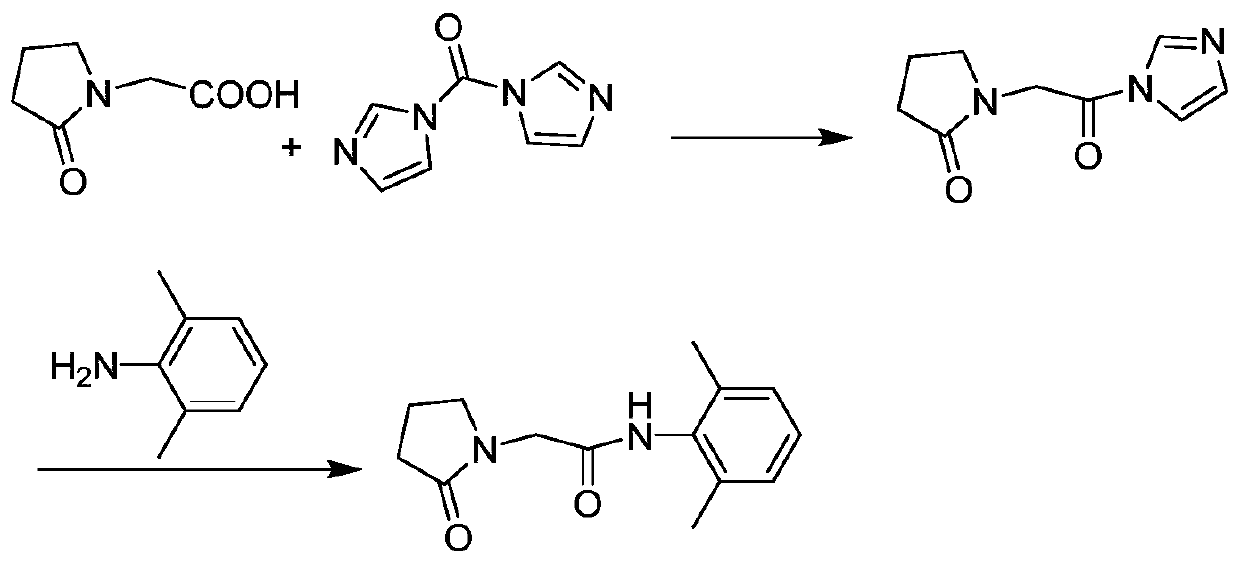
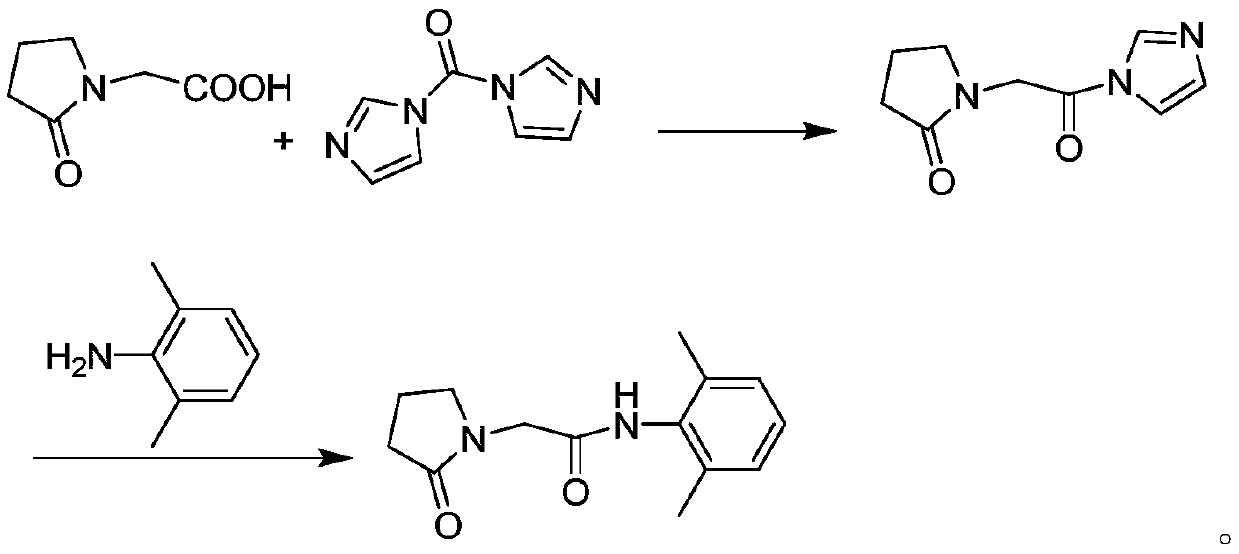
Preparation method of drug nefiracetam for treating Alzheimer's disease
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Preparation method of drug nefiracetam for treating Alzheimer's disease
The invention belongs to the field of synthesis of western medicines, and particularly discloses a preparation method of a medicine nefiracetam for treating Alzheimer's disease. According to the preparation method, carboxyl in 2-oxopyrrolidine acetic acid is activated through N',N-carbonyldiimidazole (CDI), the reaction activity is improved, the reaction rate is increased, side reactions are reduced, the obtained product is high in yield and purity, the production cost is reduced, and industrial production is facilitated.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
A kind of efficient and high atom-economical method for synthesizing imidazoline derivatives
The invention belongs to the technical field of chemical industry, and specifically relates to a method for synthesizing imidazoline derivatives with high efficiency and high atom economy. The present invention is under rare earth catalytic system, will C‑ Aryl ‑N‑ Allylamidines undergo intramolecular cyclization to imidazolines. The raw materials used in the method of the invention have wide sources or are easy to prepare, are easy to operate, can synthesize imidazoline derivatives with high yield under mild conditions, and have high reaction selectivity.
Owner:FUDAN UNIV
Method for analyzing (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol as intermediate of florfenicol
ActiveCN101941927BEasy to manufactureHigh yieldOrganic chemistryOrganic compound preparationPtru catalystBenzaldehyde
The invention relates to a method for analyzing (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol as an important intermediate of florfenicol, which is chloramphenicol spectrum antibiotic special for animals. The method comprises the following steps of: performing a reaction of methylsulfonyl benzaldehyde and nitro alcohol at 0-40 DEG C in the presence of a chiral catalyst to obtain (1R, 2R)-2-nitro-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol; and reducing the (1R, 2R)-2-nitro-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol through hydrogen to obtain the intermediate. In the invention, the compound (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol with a high e.e. value is obtained by adopting the chiral catalyst, and the compound has the advantages of strong reaction selectivity, high productivity, simple process, low cost, easy preparation of raw materials and low price, is suitable for industrial production, avoids chiral separation frequently used in industry at present and saves the raw materials and the cost. The compound (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol is used for preparing florfenicol and can reduce the preparation cost.
Owner:MASTEAM BIO TECH
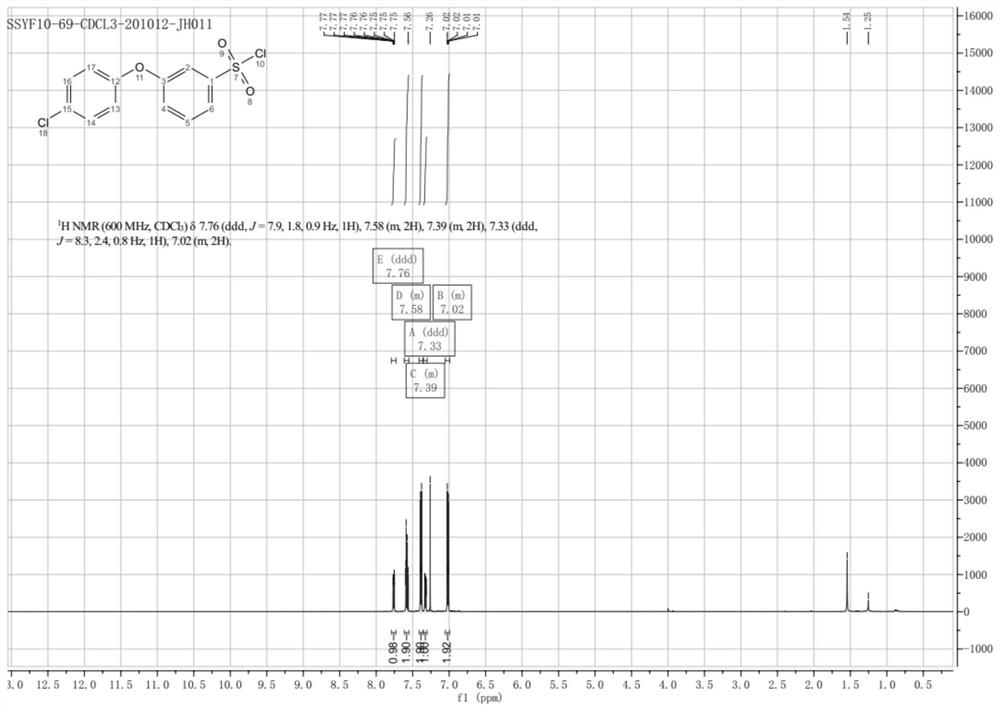
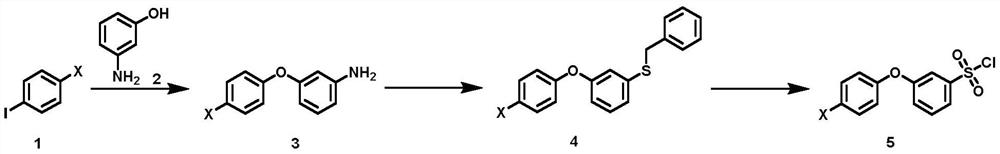
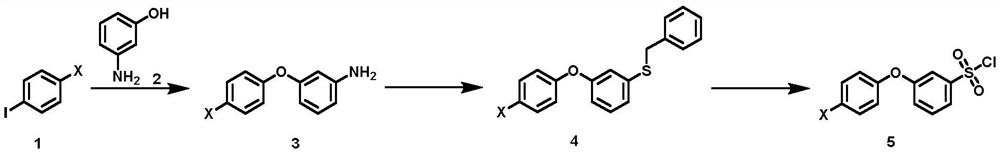
Synthesis method of 3-(halogenated phenoxy) benzene sulfonyl chloride derivative
PendingCN113563235AFew synthetic stepsLower synthesis costOrganic compound preparationSulfide preparationBenzyl chlorideBenzene
The invention discloses a synthesis method of a 3-(halogenated phenoxy) benzene sulfonyl chloride derivative. The synthesis method comprises the following steps of: by taking a p-halogen iodobenzene compound and m-hydroxyaniline which are cheap and easy to obtain as raw materials, carrying out Ullmann coupling reaction in the presence of a copper catalyst and a ligand to obtain halogenated 3-phenoxy aniline; making the intermediate react with sodium thiosulfate and benzyl chloride under the action of a diazo reagent to realize benzyl sulfhydrylation to obtain benzyl (3-(4-chlorophenoxy) phenyl) sulfane; and finally treating the benzyl (3-(4-chlorophenoxy) phenyl) sulfane by using an oxidation chlorination reagent to obtain a target compound. According to the method of the invention, through optimal design of a synthesis route, the synthesis cost of the route is reduced while the synthesis steps are shortened; and in the reaction, the operability is strong, the reaction selectivity is high, high-toxicity, high-corrosivity and high-environment-staining reagents are not used, so that the method is more suitable for large-scale expanded production.
Owner:上海毕得医药科技股份有限公司
Carbonyl starch oxidized using Fenton-like system and preparation method thereof
InactiveCN103509129BStrong oxidation abilityOvercome the problem of poor selectivity of the preparation processWater bathsFiltration
The invention discloses a carbonyl starch oxidized by a Fenton similar system, and a preparation method of the carbonyl starch. The Fenton similar system is composed of a cerous nitrate salt-a catalyst-H2O2; the catalyst is ferrous sulfate or cuprous sulfateb; starch is oxidized in aqueous phase at a temperature of 20 to 60 DEG C. The preparation method comprises following steps: (1) 30 to 50 weight portions of starch and 60 to 170 weight portions of water are mixed so as to obtain a turbid liquid, and the turbid liquid is subjected to gelatinization in water bath for 0.5 to 2h at a temperature of 50 to 70 DEG C; (2) 0.3 to 2.0 weight portions of ceric ammonium nitrate and 0.0015 to 0.0075 weight portion of the catalyst are added and mixed fully, pH value of the reaction solution is adjusted to 2.5 to 4.0, and the reaction solution is reacted for 10 to 24h at a temperature of 20 to 45 DEG C in enclosed conditions; (3) 1.5 to 10 weight portions of H2O2 is added, the temperature is adjusted to 45 to 60 DEG C, and the mixture is reacted for 3 to 7h; and (4) after that, the mixture is subjected to suction filtration, is washed for 3 times with acetone, and is dried in a vacuum oven for 12 to 24h at a temperature of 40 to 60 DEG C so as to obtain the carbonyl starch containing 10 to 20% of carbonyl and 2 to 6% of carboxyl. According to the preparation method, both carbonyl and carboxyl are introduced into starch at the same time, so that functional groups of starch molecules are increased, and application area of oxidized starch is widened.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com