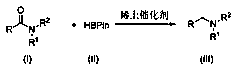
Method for synthesizing tertiary amine derivative by catalyzing hydroboration reaction of tertiary amide by rare earth
A rare earth catalysis and rare earth catalyst technology, applied in the chemical industry, can solve the problems of complex process, low reaction yield, difficult synthesis, etc., and achieve the effects of high quality, stable process and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
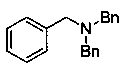
[0027] The synthesis of tribenzylamine, the chemical structure is as follows:
[0028]
[0029] Under nitrogen protection, add raw materials N , N - Dibenzylbenzamide (0.5 mmol), pinacol borane (1.2 mmol) and catalyst Y [N(SiMe 3 ) 2 ] 3 (5 mol%), reacted in xylene (3 ml) at 100 °C for 20 h, and the isolated yield of the product was 95%.
[0030] 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.49-7.47 (m, 6H), 7.38 (t, J = 7.3Hz, 6H), 7.31-7.27 (m, 3H), 3.63 (s, 6H). 13 C NMR (CDCl 3 , 125 MHz, ppm): δ139.8, 128.9, 128.4, 127.0, 58.1.
Embodiment 2
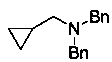
[0032] The synthesis of N,N-dibenzyl-2-cyclopropylethylamine, the chemical structure is as follows:
[0033]
[0034] Under nitrogen protection, N,N-dibenzylcyclopropylformamide (0.5 mmol), pinacol borane (1.2 mmol) and catalyst Y [N(SiMe 3 ) 2 ] 3 (10 mol%), in a mixed solution of toluene and xylene (1.5 mL+1.5 mL), reacted at 60 °C for 24 h, and the isolated yield of the product was 93%.
[0035] 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.46-7.44 (m, 4H), 7.35 (t, J = 7.3Hz, 4H), 7.28-7.25 (m, 2H), 3.71 (s, 4H), 2.38 (d, J = 6.5 Hz, 2H), 1.01-0.93(m, 1H), 0.53-0.49 (m, 2H), 0.11-0.06 (m, 2H). 13 C NMR (CDCl 3 , 125 MHz, ppm):δ 140.4, 128.8, 128.3, 126.8, 58.5, 58.4, 8.6, 4.1.
Embodiment 3
[0037] Synthesis of N,N-dibenzyl-1-(4-methoxyphenyl)methanamine, the chemical structure is as follows:
[0038]
[0039] Under nitrogen protection, add raw material N,N-dibenzyl-4-methoxybenzamide (0.5 mmol), pinacol borane (1.2 mmol) and catalyst Y [N(SiMe 3 ) 2 ] 3 (10 mol%), in toluene (3 ml), reacted at 100 ℃ for 24 h, and the isolated yield of the product was 97%.
[0040] 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.48-7.46 (m, 4H), 7.40-7.36 (m, 6H),7.31-7.27 (m, 2H), 6.93 (d, J = 8.6 Hz, 2H), 3.84 (s, 3H), 3.61 (s, 4H), 3.57(s, 2H). 13 C NMR (CDCl 3, 125 MHz, ppm): δ 158.8, 139.9, 131.7, 130.0, 128.9,128.3, 126.9, 113.8, 57.9, 57.4, 55.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com