Method for synthesizing high-yield nandrolone
A synthetic method and high-yield technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of many by-products, low quality, low yield, etc., and achieve the effect of improving yield and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Etherification reaction: In a 300L reactor equipped with stirring, meter 72 kg of absolute ethanol, 40 kg of triethyl orthoformate, and then add 75 kg of 19-norrostenedione while stirring, and stir 5 minutes; adjust the temperature in the reactor to 39-41°C, then add 0.3 kg of pyridinium hydrobromide, start the reaction, record the time for 8 hours, add 1.0 Kg of triethylamine after the reaction, cool down to 0-5°C, and Control the temperature at 0-5°C and keep stirring for 1 hour. When the reaction solution reaches neutrality, the etherification reaction is over, open the frozen brine, freeze and crystallize at 0±2°C; after the etherification reaction is terminated, release the etherification product from the reaction kettle, wash it with 98kg of absolute ethanol, Shake off filter to get wet ether compound.
[0019] 2. Reductive hydrolysis reaction: In a 1000L washed and dried reaction pot, first put 680 kg of methanol, start stirring, then put in 75 kg of ether co...
Embodiment 2
[0022] Embodiment 2: The used raw material and consumption of etherification reaction are changed, dehydrated alcohol 70kg, triethyl orthoformate 35kg, 19-norandrostenedione 70 kg, pyridinium hydrobromide 0.2 kg, triethylamine 0.6 kg;
[0023] The used raw materials and consumption of reduction hydrolysis reaction are changed into, methanol 650kg, ether compound 70kg, sodium borohydride 5kg;
[0024] The mass ratio of raw materials involved in crude product refining is 60kg of nandrolone crude product, 28kg of ethyl acetate, and 35kg of petroleum ether.
[0025] All the other are with embodiment 1.
Embodiment 3
[0026] Embodiment 3: the used raw material of etherification reaction and consumption change, dehydrated alcohol 75kg, triethyl orthoformate 45kg, 19-norandrostenedione 80 kg, pyridinium hydrobromide 0.5 kg, triethylamine 1.5 kg;
[0027] The used raw materials and consumption of reduction hydrolysis reaction are changed into, methanol 700kg, ether compound 80kg, sodium borohydride 10kg;
[0028] The mass ratio of raw materials involved in crude product refining is 65-68 kg of nandrolone crude product, 35 kg of ethyl acetate, and 45 kg of petroleum ether.
[0029] All the other are with embodiment 1.
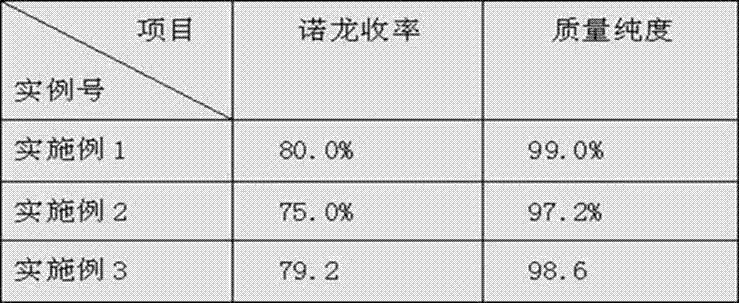
[0030] The product of embodiment 1~3 and quality purity are shown in the following table:
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com