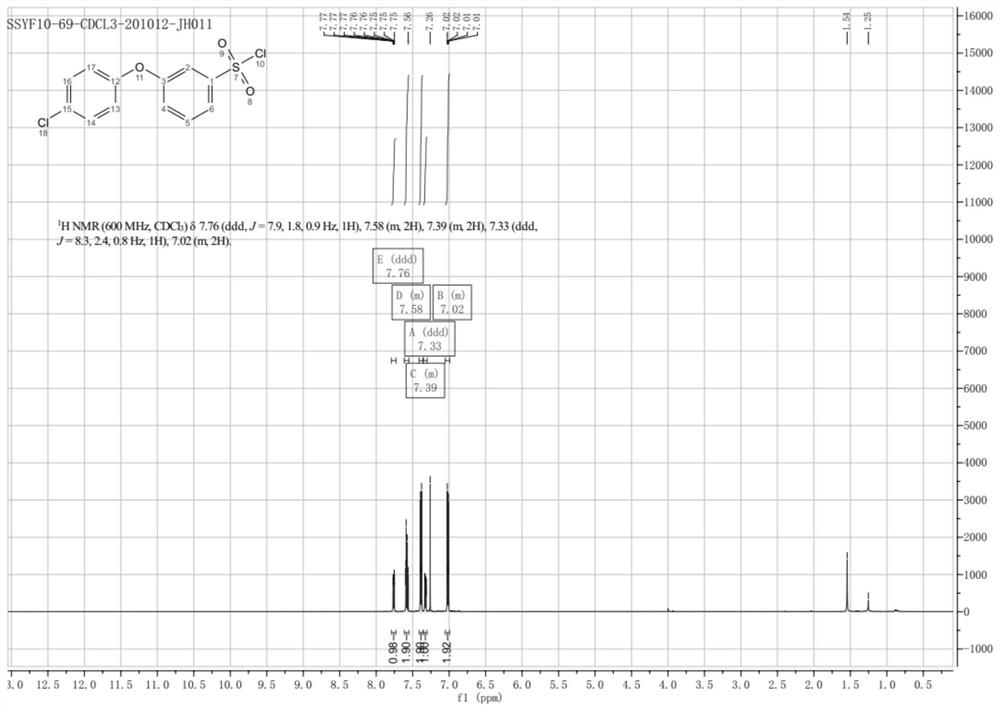
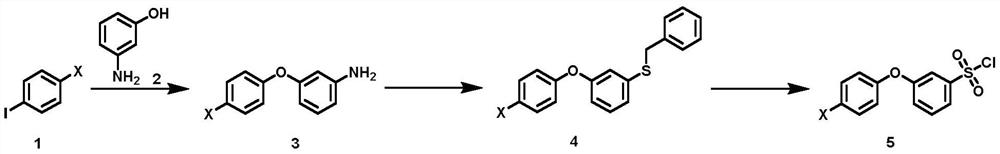
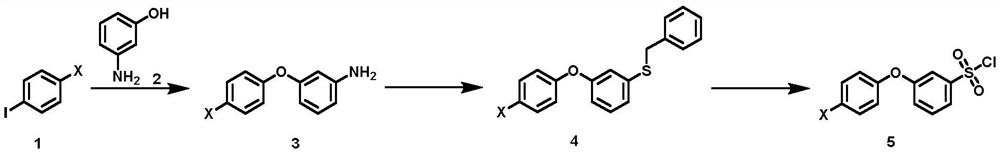
Synthesis method of 3-(halogenated phenoxy) benzene sulfonyl chloride derivative
A technology of benzenesulfonyl chloride derivatives and halogenated phenoxy groups, which is applied in the field of synthesis of 3-benzenesulfonyl chloride derivatives, can solve the problems of polluted environment, high corrosion, high toxicity of benzenesulfonyl chloride reagents, etc., and achieves reaction selection High performance, strong operability, and the effect of reducing synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The synthetic method of 3-(halogenated phenoxy) benzenesulfonyl chloride derivatives of the present invention comprises: cheap and easy-to-get p-halogenoiodobenzene compound and m-hydroxyaniline are raw materials, carry out Ullmann method in the presence of copper catalyst and ligand Coupling reaction obtains halogenated 3-phenoxyaniline intermediate, which, under the action of diazo reagent, reacts with sodium thiosulfate and benzyl chloride to realize benzyl thiolation to obtain benzyl (3-(4 -Chlorophenoxy)phenyl)sulfane intermediate, and finally treated with oxidative chlorination reagent to obtain the target compound.
[0049] A kind of synthetic method of 3-(halogenated phenoxy group) benzenesulfonyl chloride derivative, its synthetic route is as shown in formula (I):
[0050]
[0051] Wherein, X is fluorine, chlorine or bromine.
[0052] The synthetic method of described 3-(halogenated phenoxy) benzenesulfonyl chloride derivatives comprises the following steps...
Embodiment 1-17
[0082] Embodiments 1-17 describe the present invention in further detail through the synthesis of 3-(4-chlorophenoxy)benzene-1-sulfonyl chloride, which includes the following steps:
[0083] The first step: the preparation of compound 33-(4-chlorophenoxy)aniline;
[0084] The second step: the preparation of compound 41-(4-chlorophenoxy)-3-iodobenzene;
[0085] The third step: the preparation of compound 53-(4-chlorophenoxy)benzene-1-sulfonyl chloride.
Embodiment 1
[0087] The preparation of step (1) 3-(4-chlorophenoxy)aniline, its synthetic route is as follows:
[0088]
[0089]2,9-diphenyl-1,10-phenanthroline (2.77g, 8.34mmol, 0.1eq), p-chloroiodobenzene (20g, 83.40mmol, 1.0eq), m-hydroxyaniline (10.98g, 100.60mmol , 1.2eq), join in the dimethylsulfoxide of 170ml, then add tripotassium phosphate (35.6g, 167.7mmol, 2.0eq), then add copper powder (0.25g, 167.7mmol, 0.05eq), inert gas protection, React overnight at 80°C. The reaction was monitored by TLC until the reaction was complete, washed into ice water, extracted with ethyl acetate, mixed and passed through the column to obtain 15.19 g of compound 3 with a yield of 81.41% and a purity of 98.21%.
[0090] The preparation of step (2) 1-(4-chlorophenoxy group)-3-iodobenzene, its synthetic route is as follows:
[0091]
[0092] Sodium thiosulfate pentahydrate (118.62g, 477.96mmol, 7eq), benzyl chloride (60.5g, 477.96mmol, 7eq), anhydrous copper sulfate (1.09g, 6.83mmol, 0.1eq), 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com