A kind of efficient and high atom-economical method for synthesizing imidazoline derivatives
An imidazoline derivative and a technology for synthesizing imidazoline, which are applied in the chemical industry, can solve the problems of poor atom economy and substrate compatibility, harsh method conditions and high reaction temperature, and achieve simple purification, simple reaction conditions and strong reaction selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
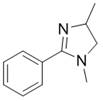
[0024] The preparation of 1,4-dimethyl-2-phenylimidazoline has the following structural formula:
[0025]
[0026] Under nitrogen protection, add raw material N-allyl-N-methyl benzamidine (0.5 mmol) and catalyst Y[N(SiMe 3 ) 2 ] 3 (10 mol%), N-methylallylamine (1 mL), reacted at 25 °C for 12 h, and the isolated yield of the product was 96%.
[0027] 1 H NMR (400 MHz, CDCl 3 ): δ 7.54-7.53 (m, 2H), 7.38-7.37 (m, 3H), 4.18-4.09 (m, 1H), 3.57 (t, J = 8.0, 1H), 2.98 (t, J = 8.0 Hz, 1H), 2.76 (s, 3H), 1.32 (d, J = 6.6 Hz, 3H).
Embodiment 2
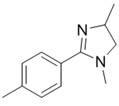
[0029] The preparation of 1,4-dimethyl-2-p-methylphenylimidazoline has the following structural formula:
[0030]
[0031] Under nitrogen protection, add raw material N-allyl-N-methyl-p-methyl benzamidine (0.5 mmol) and catalyst Y[N(SiMe 3 ) 2 ] 3 (8 mol%), N-methylallylamine (1 mL), reacted at 25 °C for 12 h, and the isolated yield of the product was 85%.
[0032] 1 H NMR (400 MHz, CDCl 3 ): δ 7.45-7.43 (m, 2H), 7.20-7.18 (m, 2H), 4.17-4.08 (m, 1H), 3.57 (t, J = 9.5 Hz, 1H), 2.97 (t, J = 8.7 Hz, 1H ), 2.77 (s,3H), 2.36 (s, 3H), 1.32 (d, J = 6.6 Hz, 3H).
Embodiment 3
[0034] The preparation of 1,4-dimethyl-2-p-chlorophenylimidazoline has the following structural formula:
[0035]
[0036] Under nitrogen protection, add raw material N-allyl-N-methyl p-chlorobenzamidine (0.5 mmol) and catalyst Y[N(SiMe 3 ) 2 ] 3 (10 mol%), N-methylallylamine (1 mL), reacted at 25 °C for 10 h, and the isolated yield of the product was 92%.
[0037] 1 H NMR (400 MHz, CDCl 3 ): δ 7.50-7.48 (m, 2H), 7.38-7.36 (m, 2H), 4.18-4.08 (m, 1H), 3.58 (t, J = 9.5 Hz, 1H), 2.98 (t, J = 8.8 Hz, 1H), 2.76 (s,3H), 1.32 (d, J = 6.6 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com