Preparation method of citral
A technology of citral and compound, applied in the field of preparation of citral, can solve the problems of difficult separation, low yield, large amount of catalyst, etc., and achieves high economy and selectivity, simple operation steps, high yield and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: the preparation of citral (Ⅰ)
[0086]
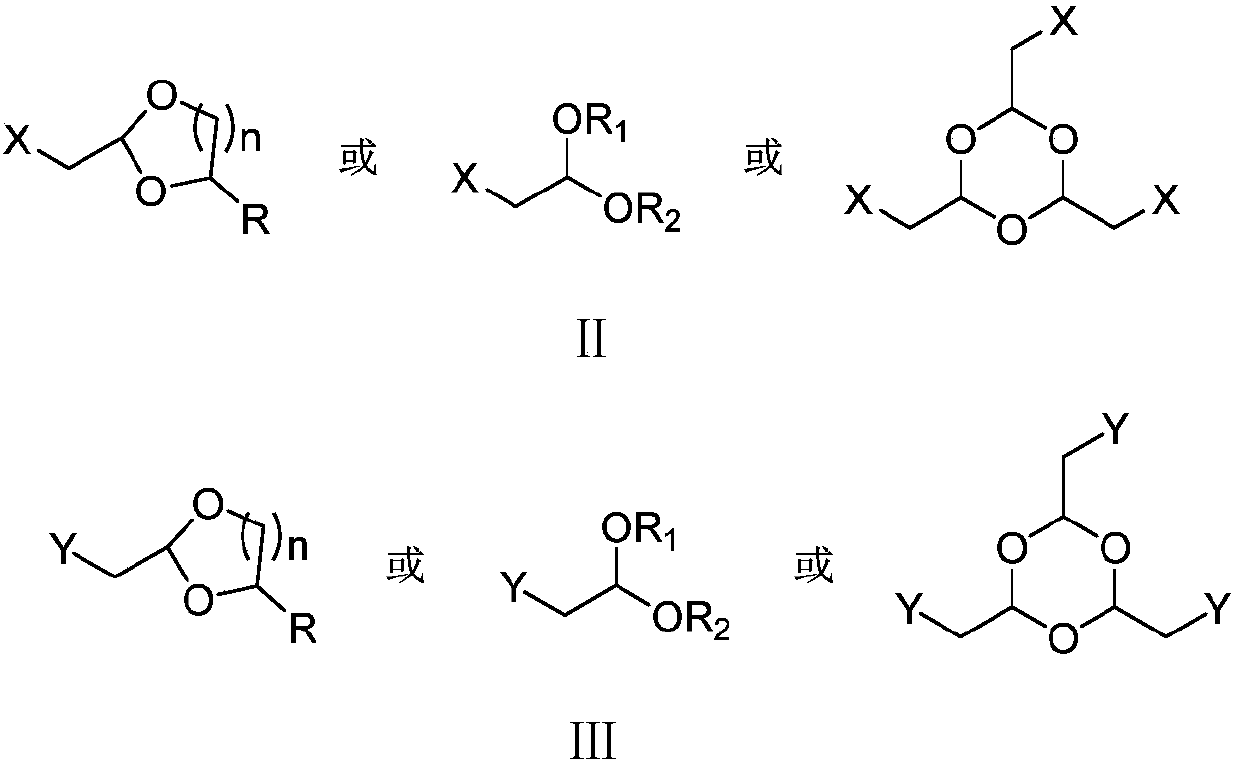
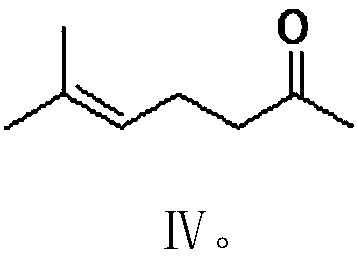
[0087] Under nitrogen protection, add 100 grams of tetrahydrofuran, 5.3 grams (0.22 moles) of magnesium powder, 1.8 grams of bromoacetaldehyde dimethyl acetal ( II 1 ), 0.05 gram of iodine, after stirring for 15 minutes at 30-40°C to initiate the reaction, 32.0 grams (a total of 0.2 moles) of bromoacetaldehyde dimethyl acetal (II 1 ) and 100 grams of tetrahydrofuran, drop it in 2 hours, then stir and react at 35-40°C for 1 hour to obtain III 1 without separation, cooled to 0-5°C, kept at 10-15°C, added dropwise 25.0 g (0.2 moles) of 6-methyl-5-hepten-2-one (Ⅳ), and added dropwise in 1 hour , after which the reaction was stirred at 10-15° C. for 2 hours. Distill under reduced pressure to recover tetrahydrofuran, add 100 grams of water and 100 grams of dichloromethane to the obtained residue, acidify with 50% acetic acid until the pH of the system is 4.0-4.5, stir at 30-35 ° C for 2 hours, and let it stand Layere...
Embodiment 2
[0091] Embodiment 2: the preparation of citral (Ⅰ)
[0092]
[0093] Under nitrogen protection, add 100 grams of tetrahydrofuran (THF), 5.3 grams (0.22 moles) of magnesium powder, 2.2 grams of bromoacetaldehyde diethyl acetal ( II 2 ), 0.02 gram of iodine, after stirring for 15 minutes at 30-40°C to initiate the reaction, 37.0 grams (a total of 0.2 moles) of bromoacetaldehyde diethyl acetal (Ⅱ) was added dropwise between 30-35°C 2 ) and 100 grams of tetrahydrofuran, drop it in 2 hours, then stir and react at 35-40°C for 1 hour to obtain III 2 . Cool to 0-5°C, keep the temperature at 10-15°C, add 25.0 g (0.2 moles) of 6-methyl-5-hepten-2-one (IV) dropwise, dropwise for 1 hour, then 10-15 The reaction was stirred at °C for 2 hours. Recover tetrahydrofuran by distillation under reduced pressure, add 100 g of water and 100 g of dichloromethane to the resulting residue, acidify with 50% aqueous phosphoric acid until the pH of the system is 4.0-4.5, stir at 20-25°C for 2 hour...
Embodiment 3
[0094] Embodiment 3: the preparation of citral (Ⅰ)
[0095]
[0096] Step (1): 2-triphenylphosphinoacetaldehyde dimethyl acetal bromide (Ⅲ 3 ) preparation
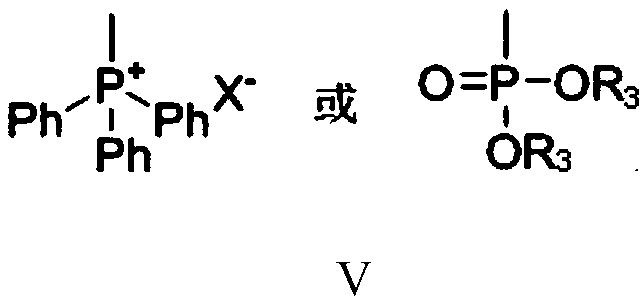
[0097] Under nitrogen protection, add 180 grams of acetonitrile, 33.8 grams (0.2 moles) of bromoacetaldehyde dimethyl acetal (II 1 ), 52.5 grams (0.2 moles) of triphenylphosphine, stirred and reacted at 60-65°C for 3 hours, cooled to 10-15°C, filtered, and obtained 85.3 grams of formula III after drying the filter cake 3 Compound, the yield is 98.9%, and the liquid phase purity is 99.5%.
[0098] Step (2): Preparation of Citral (I)
[0099] Under nitrogen protection, add 100 grams of N,N-dimethylformamide, 7.5 grams (0.11 moles) of solid sodium ethylate, 43.1 grams (0.1 moles) Embodiment 3 step (1) method gained formula III 3 Compound, cooled, kept between 10°C and 15°C, added dropwise 12.6 g (0.1 mol) of 6-methyl-5-hepten-2-one (Ⅳ), added dropwise for 1 hour, then stirred at 15-20°C React for 3 hours. Recover N,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com