Method for analyzing (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol as intermediate of florfenicol
A synthesis method and florfenicol technology are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problems of difficult industrial production, high waste water treatment costs, and unfavorable control costs, and achieve easy preparation. , The effect of reducing the preparation cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
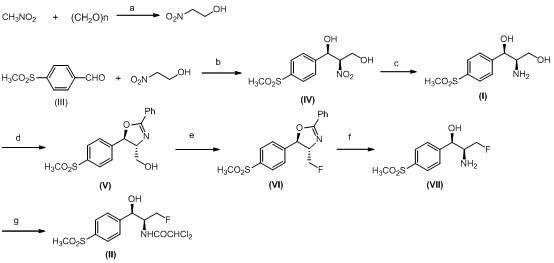
[0037] 1. Synthesis of nitroethanol:
[0038] Add paraformaldehyde (40 g, 1.3 mol), 400 g nitromethane (400.0 g, 6.6 mol), sodium fluoride (2.0 g, 34.4 mmol), and isopropanol (120 ml) to a 1000 ml round bottom flask. , Stirring, reacting at 40 ℃ for about 20 h, filtering to remove sodium fluoride, rotary evaporation to remove excess nitromethane and solvent isopropanol, to obtain a colorless transparent liquid 64.0 g, a yield of 62%. Among them, nitromethane and isopropanol can be recycled and put into the reaction again.
Embodiment 2
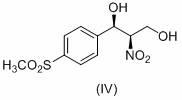
[0040] 2. Synthesis of (1R,2R)-2-nitro-1–(4-(methylsulfonyl)phenyl)-1,3-propanediol (IV) by catalyst 1:
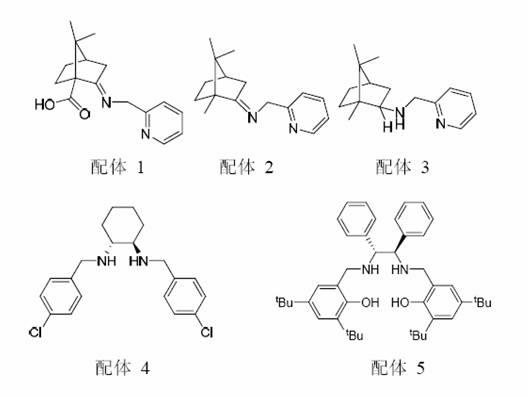
[0041] 2.1 Synthesis of chiral catalyst ligand 1:
[0042] Add (1S)-(+)-ketopine acid (4.2 g, 23.0 mmol), 2-aminomethylpyridine (2.6 g, 24.4 mmol), BF to the 250 ml reaction flask 3 ·Et 2 O (0.4 ml), CHCl 3 (150 ml), N 2 Heat and reflux under protection for 4 h. After stopping the reaction, the reaction solution was concentrated under reduced pressure and crystallized in a mixed solvent of n-hexane / dichloromethane (volume ratio 3:1) to obtain 5.0 g of ligand 1 , The yield is 80%. [α] 20 D = + 64.8 (c 1.11, CHCl 3 ).
[0043] 2.2 Synthesis of chiral catalyst 1:
[0044] Add absolute ethanol (200 ml) and the ligand prepared above (4.9 g, 18.0 mmol) to a 1000 ml reaction flask, stir to dissolve and add Cu(OAc) 2 ·H 2 O (3.2 g, 16.3 mmol), and the mixture was stirred for 1 h. The resulting solution was the prepared chiral catalyst solution and was ready for use.
[0045] 2.3 Synt...
Embodiment 3
[0048] 3. Catalyst 2 synthesis (1R,2R)-2-nitro-1–(4-(methylsulfone)phenyl)-1,3-propanediol (IV):
[0049] 3.1 Synthesis catalyst 2:
[0050] Add absolute ethanol (200 ml) to the 1000 ml reaction flask, and the ligand prepared above 1 (4.9 g, 18.0 mmol), stir to dissolve and add Cu(OTf) 2 (5.8 g, 16.3 mmol), the mixture was stirred for 1 h, and the obtained solution was the prepared chiral catalyst solution, ready for use.
[0051] 3.2 Synthesis of catalyst 2 (1R,2R)-2-nitro-1–(4-(methylsulfone)phenyl)-1,3-propanediol (IV):
[0052] The experiment operation is the same Example 2.3 . 11.2 g of the product was obtained, the yield was 25%, and the e.e. value determined by HPLC was 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com