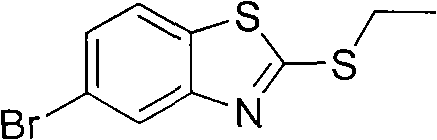
Preparation method of 2-methylthio-5-benzothiazole
A technology of bromobenzothiazole and mercaptobenzothiazole, which is applied in the field of synthesis of organic compounds, can solve the problems of large discharge of three wastes, long synthesis route, and low reaction yield, and achieve less discharge of three wastes, reduced corrosion, and high reaction yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
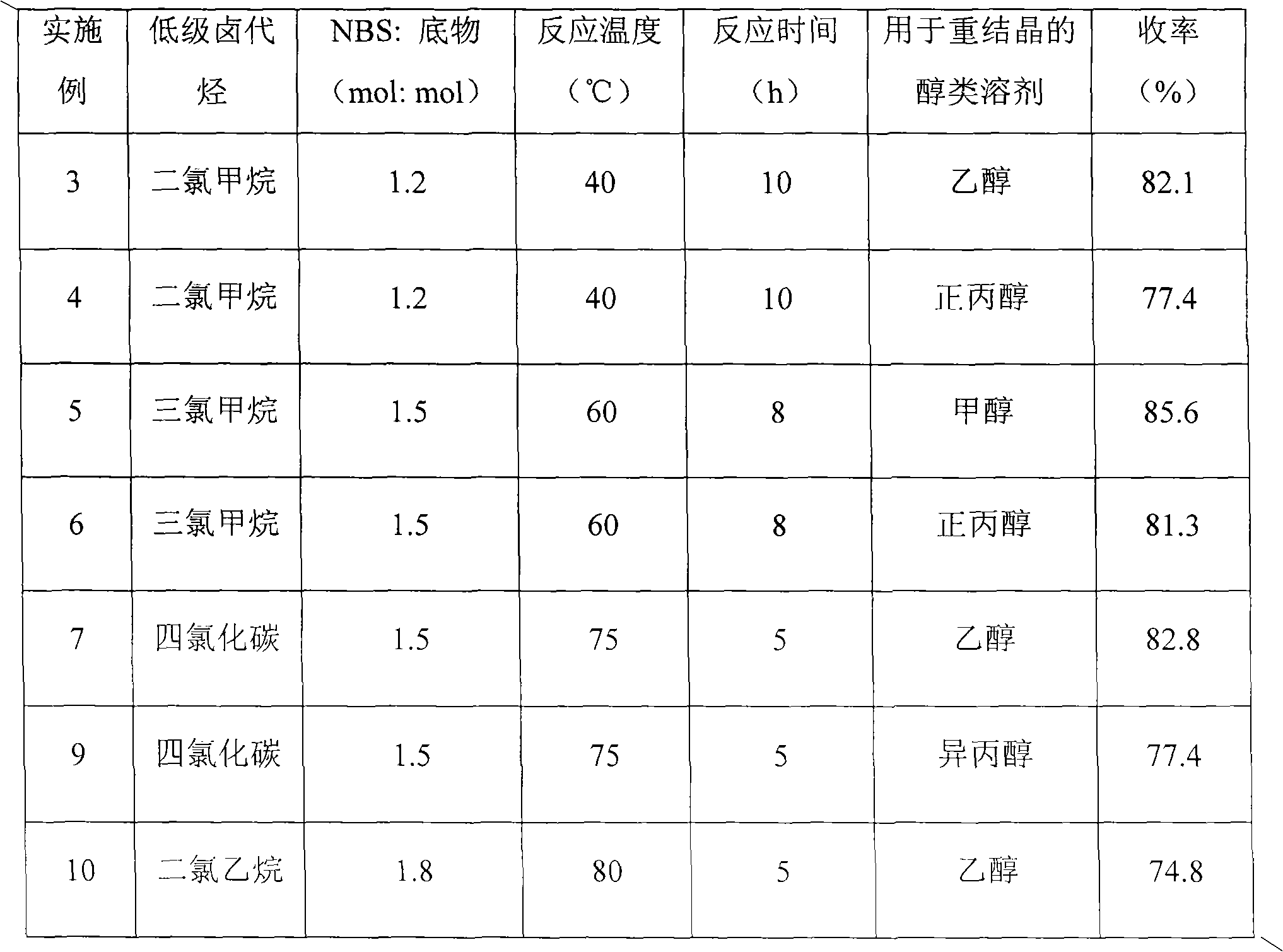
[0025] Embodiment 1, a kind of preparation method of 2-ethylthiobenzothiazole, take 2-mercaptobenzothiazole as starting material, carry out the following steps successively:
[0026] A 1000mL three-neck round bottom flask was equipped with a mechanical stirring paddle, a spherical condenser, and a constant pressure dropping funnel. Add 250mL of industrial ethanol and 160mL of 20% (mass concentration) sodium hydroxide aqueous solution, and heat to reflux. 100 g of 2-mercaptobenzothiazole was thrown into the flask at one time, and stirred to obtain a pale yellow clear reaction liquid. Measure 50 mL of ethyl bromide and add it dropwise to the flask within 1 hour. After the dropwise addition, continue to react for 2 hours. It is found that the raw materials have been consumed by TLC monitoring. After the reaction solution was cooled to room temperature, it was separated into two phases. After the lower organic phase was separated, 50 mL of dichloromethane was added, washed with wa...
Embodiment 2
[0027] Embodiment 2, a preparation method of 2-ethylthio-5-bromobenzothiazole, using 2-ethylthiobenzothiazole as a starting material, the following steps are carried out in sequence:
[0028] Dissolve 2-ethylthiobenzothiazole (9.77 g, 50.0 mmol) in 100 mL CHCl 3 , transferred to a 250mL three-necked flask equipped with a mechanical stirring paddle, a thermometer, and a spherical condenser, started stirring, and refluxed at 60°C. Commercialized NBS (13.4 g, 75.0 mmol) was added into the flask at one time, and the reaction solution gradually turned yellow, orange, orange red until dark red. GC detection, 9h to stop the reaction. After the resulting reaction solution was cooled to room temperature, filter it to remove succinimide as a by-product, and wash the obtained filtrate with saturated sodium bicarbonate solution (30mL×3) until the reddish-brown color disappeared, and the organic phase was basically pale. Until it becomes yellow (that is, remove a small amount of bromine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com