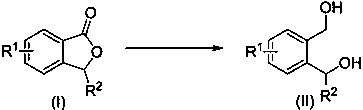Method for synthesizing o-benzenedicarbinol derivative
A technology for ortho-phthalic alcohol and derivatives, which is applied in the field of synthesizing ortho-phthalic alcohol derivatives, can solve the problems of difficult source of reaction raw materials, high requirements for reaction equipment, expensive hydrogenation catalysts, etc., and meets the requirements of easy transportation, storage, and equipment. The effect of low, simple preparation process and product separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of o-phthalmethanol:
[0024]
[0025] Put phthalide (0.4 mmol), potassium hydroxide (0.4 mmol), phenylsilane (1.2 mmol) and tetrahydrofuran (4 mL) into the reaction flask, react at 66°C for 4 h, stop the reaction, add a small amount of alcohol dropwise to quench the reaction, After separation, 54 mg of o-phthalamide was obtained, with a yield of 98%.
Embodiment 2
[0026] Example 2 Put phthalide (0.4 mmol), sodium hydroxide (0.4 mmol), diphenylsilane (1.6 mmol) and tetrahydrofuran (4 mL) into the reaction flask, react at 66°C for 4 h, stop the reaction, add a small amount of The reaction was quenched with alcohol, and 44 mg of o-phthalyl dimethanol was obtained after separation, with a yield of 80%.
Embodiment 3
[0027] Example 3 Put phthalide (0.4 mmol), potassium hydroxide (0.4 mmol), triphenylsilane (1.6 mmol) and tetrahydrofuran (4 mL) into the reaction flask, react at 66°C for 6 h, stop the reaction, add a small amount of The reaction was quenched with alcohol, and 43 mg of o-phthalyl dimethanol was obtained after separation, with a yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com