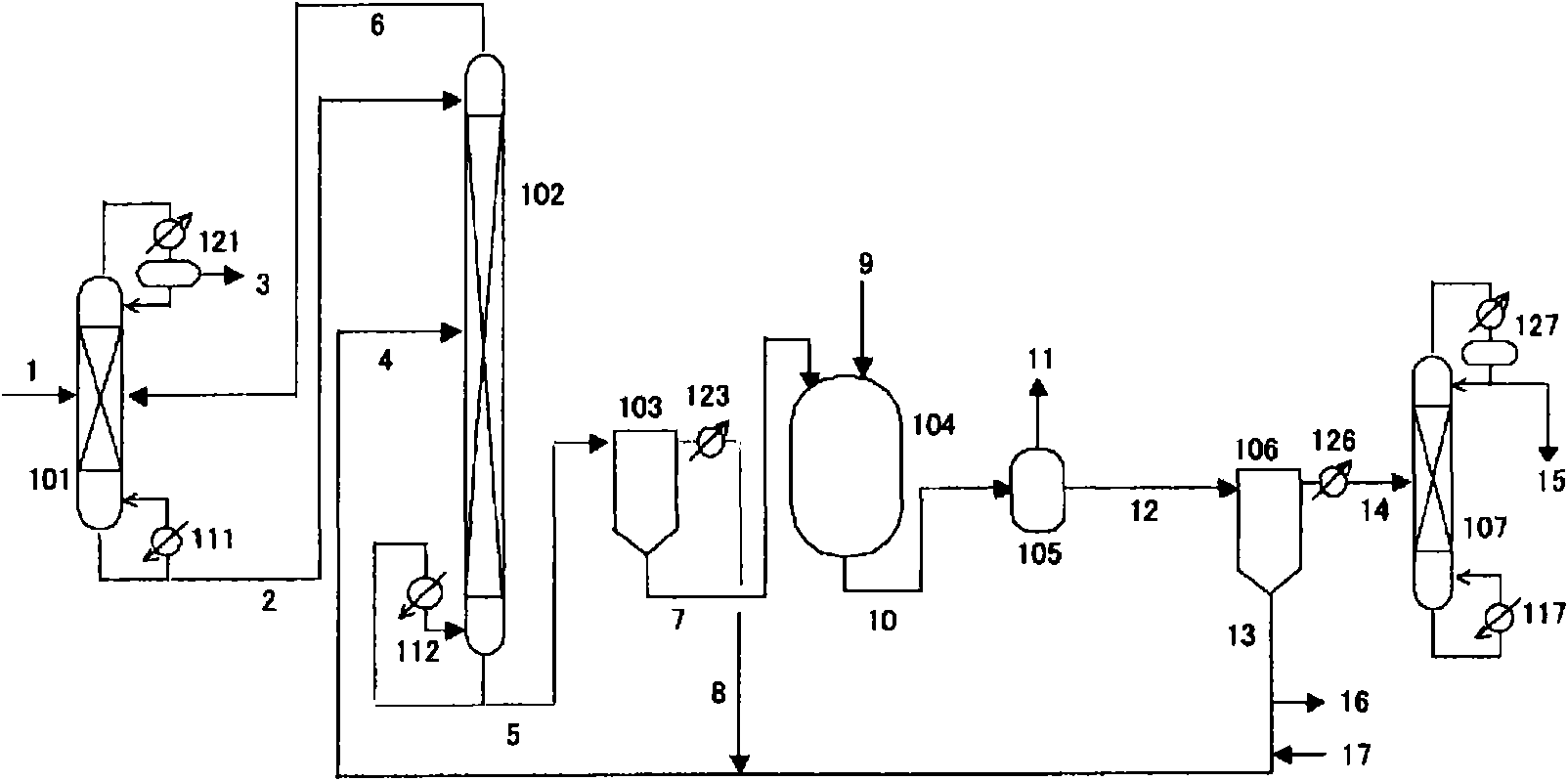
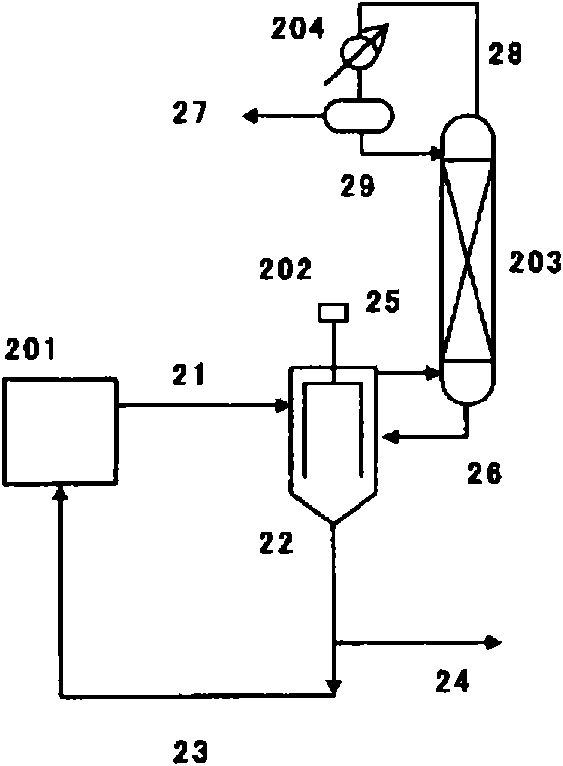
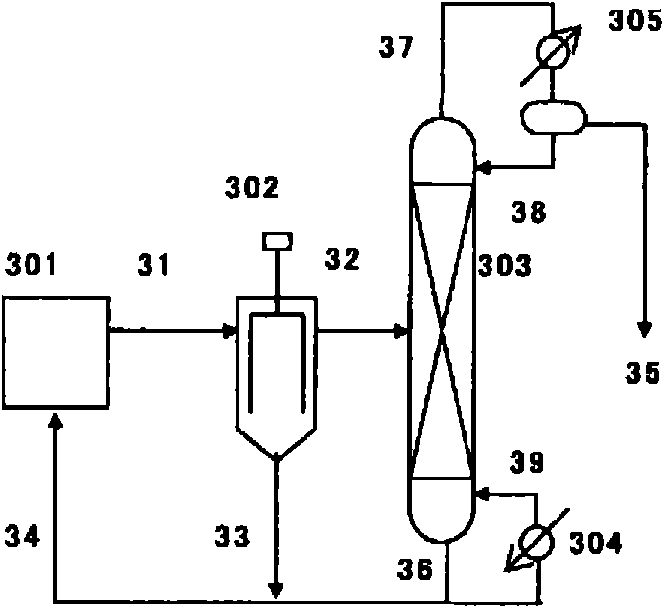
Method for producing isocyanate
A manufacturing method and technology of isocyanate, applied in the field of isocyanate manufacturing, can solve the problems of polyurethane products such as weather resistance and adverse effects of heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0181] Hereinafter, the present invention will be specifically described based on examples, but the scope of the present invention is not limited to these examples.
[0182]
[0183] 1) NMR analysis method
[0184] Device: JNM-A400 FT-NMR system manufactured by Nippon Electronics Co., Ltd., Japan
[0185] (1) 1 H and 13 Preparation of samples for C-NMR analysis
[0186] Weigh about 0.3 g of the sample solution, add about 0.7 g of deuterated chloroform (manufactured by Aldrich, U.S., 99.8%) and 0.05 g of tetramethyltin (manufactured by Wako Pure Chemical Industries Ltd. of Japan, Wako Class I) as an internal standard substance, The uniformly mixed solution was used as a sample for NMR analysis.
[0187] (2) Quantitative analysis method
[0188] Each standard substance is analyzed, a calibration curve is prepared, and the analysis sample solution is quantitatively analyzed based on the prepared calibration curve.
[0189] 2) Liquid chromatography analysis method
[0190...
reference example 1
[0210] [Reference Example 1] Production of bis(3-methylbutyl)carbonate
[0211] ・Step (I-1): Production of dialkyltin catalyst
[0212]Add 625 g (2.7 mol) of di-n-butyltin oxide (manufactured by Japan Sankyo Organic Synthesis Co., Ltd.) and 2020 g (22.7 mol) of 3-methyl-1-butanol (manufactured by Japan Kuraray Co., Ltd.) into an eggplant-shaped flask with a capacity of 5000 mL. ). The flask was installed on an evaporator (manufactured by Japan Shibata Corporation, R-144), which was connected with an oil bath with a temperature regulator (manufactured by Japan Masuda Physical and Chemical Industry Corporation, OBH-24), a vacuum pump (Japan ULVAC Corporation Manufactured, G-50A) and vacuum controller (manufactured by Okano Manufacturing Co., Ltd., VC-10S). The outlet of the purge valve of the evaporator is connected with the pipeline flowing nitrogen at normal pressure. Close the vent valve of the evaporator, after reducing the pressure in the system, slowly open the vent val...
reference example 2
[0215] [Reference Example 2] Manufacture of dibutyl carbonate
[0216] ・Step (II-1): Production of dialkyltin catalyst
[0217] 692 g (2.78 mol) of di-n-butyltin oxide and 2000 g (27 mol) of 1-butanol (manufactured by Wako Pure Chemical Industries, Ltd.) were placed in an eggplant-shaped flask with a volume of 3000 mL. The flask containing this mixture as a white slurry was mounted on an evaporator connected to an oil bath with a temperature regulator, a vacuum pump and a vacuum controller. The outlet of the purge valve of the evaporator is connected with the pipeline flowing nitrogen at normal pressure. Close the vent valve of the evaporator, after reducing the pressure in the system, slowly open the vent valve, circulate nitrogen in the system, and return to normal pressure. The temperature of the oil bath was set at 126° C., the flask was immersed in the oil bath, and the evaporator was started to rotate. After rotating and heating at normal pressure for about 30 minutes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com