Preparation method of drug nefiracetam for treating Alzheimer's disease
A technology of nefiracetam and a synthesis method, which is applied in the field of western medicine synthesis, can solve the problems of long reaction route of nefiracetam, expensive raw materials, low product purity, etc., and achieves high yield, easy reaction process, and reaction selectivity. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention discloses a preparation method of nefiracetam, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. It needs to be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention, and relevant personnel can obviously make changes without departing from the content, spirit and scope of the present invention. Changes or appropriate changes and combinations are made to the content described herein to realize and apply the technology of the present invention.
[0024] In the present invention, unless otherwise specified, the scientific and technical terms used herein have the meanings commonly understood by those skilled in the art.
Embodiment 1
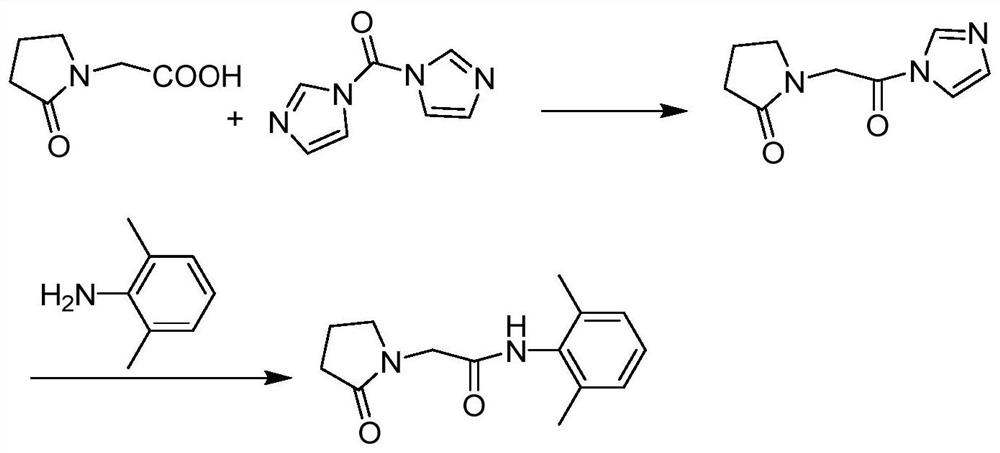
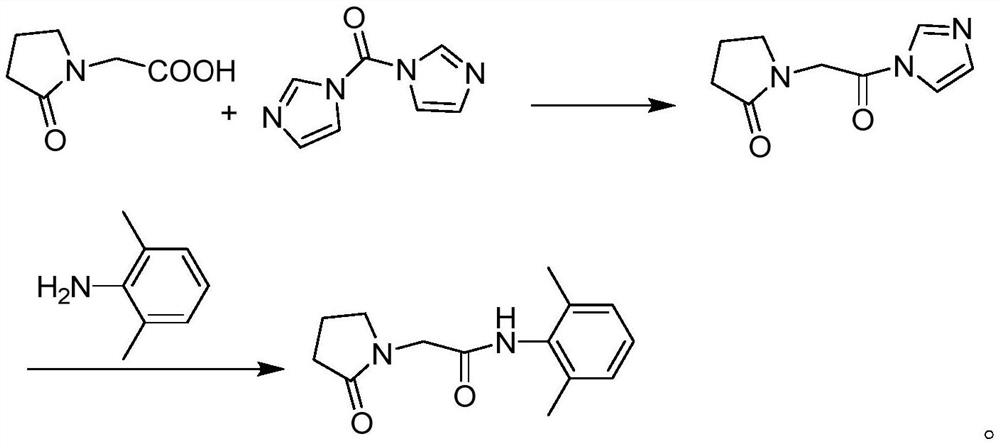
[0027] In 250 mL of tetrahydrofuran, add 14.3 g (about 0.1 mol) of 2-oxopyrrolidine acetic acid, 17.7 g (about 0.11 mol) of CDI and 20.2 g (about 0.2 mol) of triethylamine, heat to 40 ° C, and react for 1.5 hours. obtaining a solution containing the intermediate compound;
[0028] Add 13.3 grams (about 0.11mol) of 2,6-dimethylaniline directly into the above solution, keep it warm for half an hour, monitor the reaction progress by TLC, add 150mL water for extraction after the reaction is completed, and wash the organic phase twice with saturated sodium chloride water , dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and subjected to column chromatography, the eluate was concentrated to remove the solvent, and dried in vacuo to obtain 24.0 g (about 0.0974 mol) of nefiracetam product with an HPLC purity of 99.81%.
Embodiment 2
[0030] In 250 mL of dichloromethane, add 14.3 g (about 0.1 mol) of 2-oxopyrrolidine acetic acid, 17.7 g (about 0.11 mol) of CDI and 23.7 g (about 0.3 mol) of pyridine, heat to 35 ° C, and react for 1.5 hours. obtaining a solution containing the intermediate compound;
[0031] Add 14.5 g (about 0.12 mol) of 2,6-dimethylaniline directly into the above solution, keep it warm for half an hour, monitor the reaction progress by TLC, add 150 mL of water for extraction after the reaction is completed, and wash the organic phase twice with saturated sodium chloride water , dried over anhydrous magnesium sulfate, concentrated under reduced pressure, dissolved in ethanol, cooled and crystallized at 0°C, filtered, and vacuum-dried to obtain 23.9 g (about 0.097 mol) of nefiracetam product with an HPLC purity of 99.88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com