Preparation method of cortisone acetate
A technology for cortisone acetate and epihydrocortisone, applied in the field of steroid drug preparation, can solve problems such as unfavorable applications, and achieve the effects of simplifying production operations, reducing production costs, and reducing reaction impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
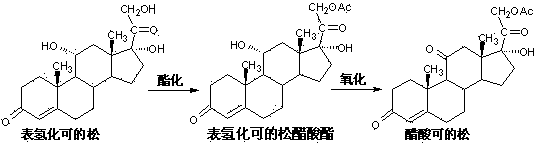
[0025] Acetylation reaction: Add 20 g of epihydrocortisone, 5.6 g of barium acetate, and 120 ml of glacial acetic acid to the reaction bottle in sequence, and stir at 20° C. for 0.5 hour. Then the temperature was lowered to 15°C, and 14ml of acetic anhydride was added dropwise. After the dropwise addition was completed, the reaction was maintained at 25° C. to 30° C. for 8 hours.
[0026] Oxidation reaction: Cool down the acetylation reaction solution after the above heat preservation reaction to 10°C, add dropwise a solution of 0.8g sodium bromide dissolved in 80ml sodium hypochlorite solution with a sodium hypochlorite content ≥ 8.0% in terms of available chlorine. After the addition is complete, keep the reaction at 13°C to 15°C for 3 hours. Slowly add the reaction solution into 1600ml of ice water pre-cooled to 0°C-5°C. After the addition is complete, continue to stir at 0°C-5°C for 0.5 hour, filter with suction, wash with water, and drain to obtain crude cortisone acetat...
Embodiment 2
[0028] Acetylation reaction: Add 20 g of epihydrocortisone, 3.6 g of barium acetate, and 80 ml of glacial acetic acid to the reaction bottle in sequence, and stir at 20° C. for 0.5 hour. Then the temperature was lowered to 15°C, and 40ml of acetic anhydride was added dropwise. After the dropwise addition was completed, the reaction was maintained at 25° C. to 30° C. for 6 hours.
[0029] Oxidation reaction: Cool down the acetylation reaction solution after the above heat preservation reaction to 10°C, add dropwise a solution of 0.4g sodium bromide dissolved in 40ml sodium hypochlorite solution with a sodium hypochlorite content ≥ 8.0% in terms of available chlorine. After the addition is complete, keep the reaction at 13°C to 15°C for 4 hours. Slowly add the reaction solution into 1600ml of ice water pre-cooled to 0°C-5°C. After the addition is complete, continue to stir at 0°C-5°C for 0.5 hour, filter with suction, wash with water, and drain to obtain crude cortisone acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com