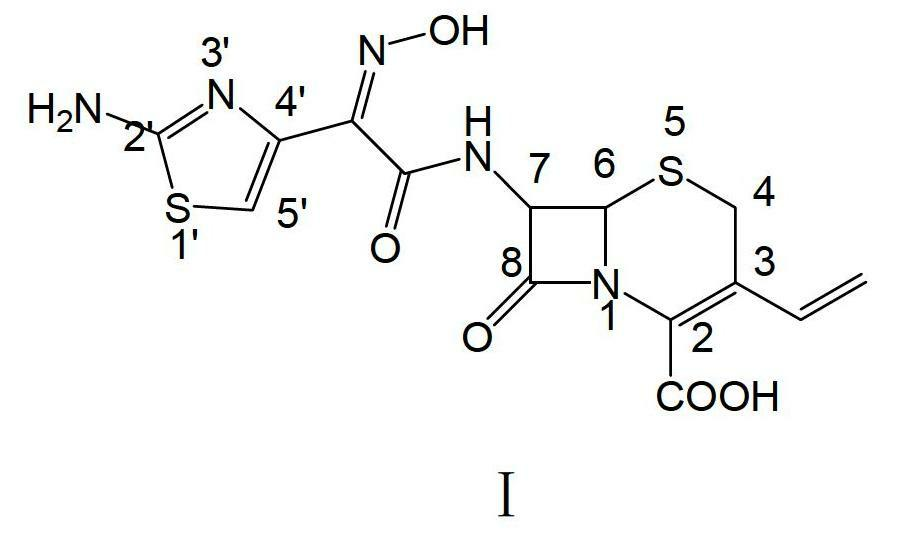
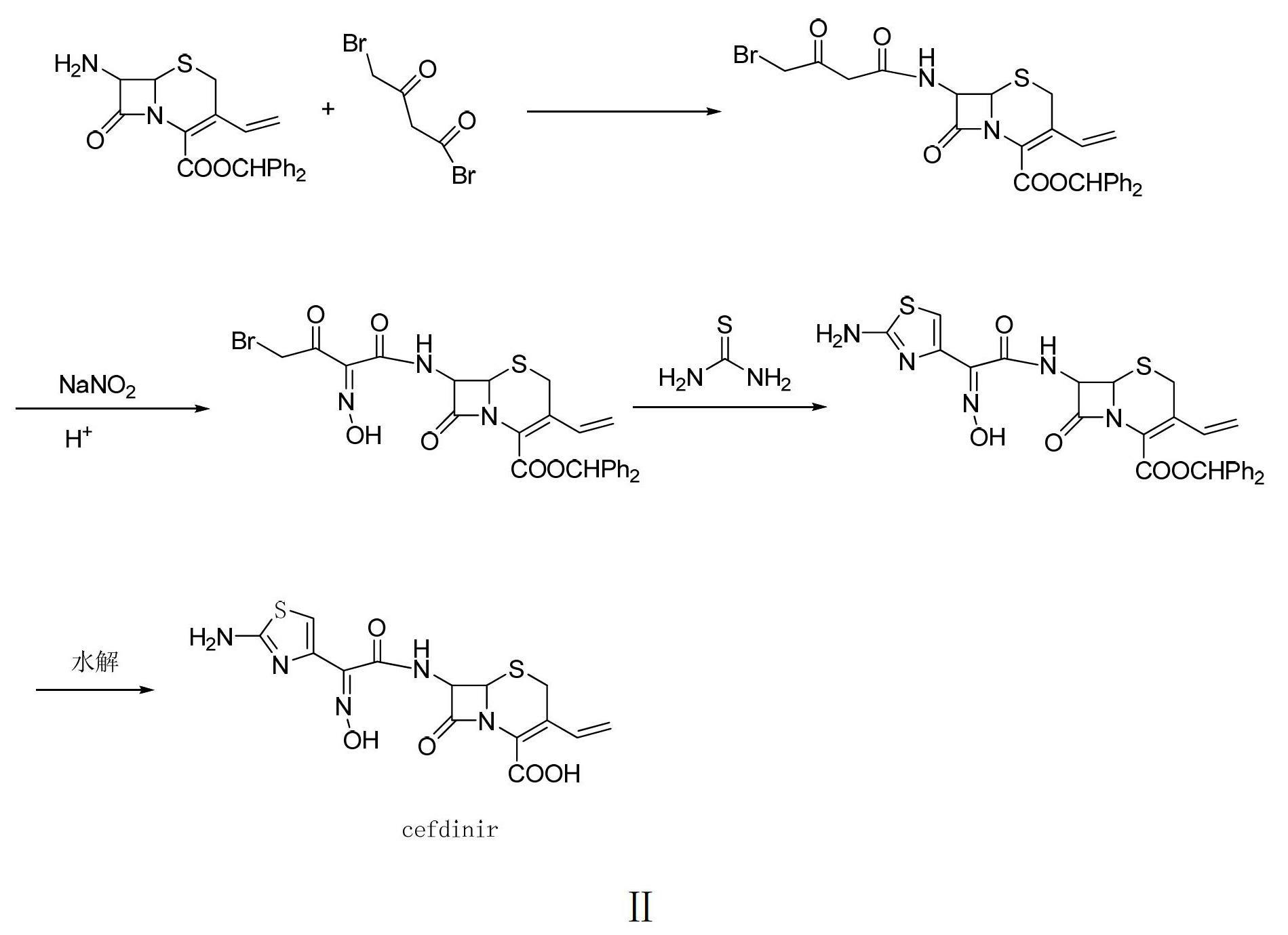
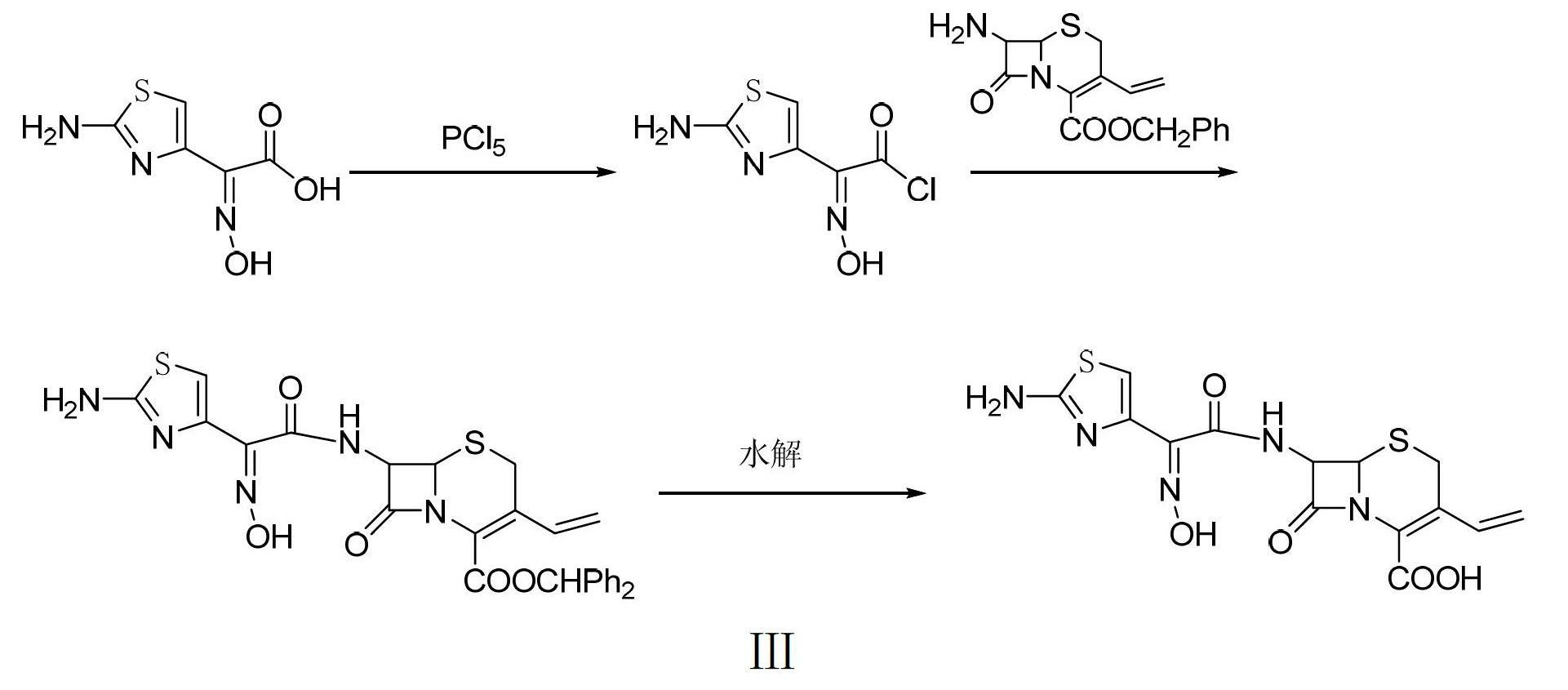
Preparation method of cefdinir
The technology of cefdinir and organic solvent is applied in the field of synthesis of cephalosporins, and can solve the problems of dark color of cefdinir product, not using low temperature conditions, affecting reaction efficiency, etc., so as to improve solvent recovery rate, reduce reaction impurities, Reduce the effect of hydrolysis side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 10kg of tetrahydrofuran and 6kg of purified water were added to the 50L reactor. The temperature was adjusted to 15±2°C, and 7-AVCA (1.0kg, 4.42mol) and T15-AE active ester (2.1kg, 5.55mol) were put into the reactor. A mixture of triethylamine (0.45kg, 4.46mol) and 2L of tetrahydrofuran was added dropwise. During the dropping process, the dropping speed is controlled so that the reaction temperature T=10±2°C and the pH value is maintained between 8.0 and 8.5. The dropwise addition is completed within 3~4h, and the temperature is kept at 10~20°C for 4~5h. After the reaction solution was dissolved, samples were taken in the control panel to detect the residue of 7-AVCA. When the residual 7-AVCA was less than 1.0%, the reaction was stopped.
[0047] 5 kg of tert-butyl acetate and 4 kg of purified water were added to the system. Leave to layer (the upper layer is lighter than the lower layer). Add 4kg tert-butyl acetate to the obtained aqueous phase, leave to stand and...
Embodiment 2
[0054] Add 10kg of acetone and 5kg of purified water into the 50L reactor. The temperature was adjusted to 20±2°C, and 7-AVCA (1.0kg, 4.42mol) and T15-AE active ester (2.0kg, 5.28mol) were put into the reactor. A mixture of tri-n-butylamine (0.9kg, 4.86mol) and 2L of acetone was added dropwise. During the dropping process, the dropping rate was controlled so that the reaction temperature was 20±2°C and the pH value was maintained between 8.0 and 8.5. The dropwise addition is completed within 3~4h. After the dropwise addition, keep warm at 20±2°C for 4~5h. After the reaction solution was dissolved, samples were taken in the control panel to detect the residue of 7-AVCA. When the residual 7-AVCA is less than 1.0%, stop the reaction.
[0055] 5 kg of propyl acetate and 4 kg of purified water were added to the system. Let stand to layer. Separate the layers, separate the water phase, add 4kg propyl acetate, and stir. Stand to separate layers, combine two propyl acetate phas...
Embodiment 3
[0059] Add 8kg dioxane and 4Kg purified water in the 50L reactor. The temperature was adjusted to 10±2°C, and 7-AVCA (1.0kg, 4.42mol) and T15-AE active ester (1.8kg, 4.86mol) were put into the reactor. A mixture of dicyclohexylamine (0.90kg, 4.98mol) and 2L of dioxane was added dropwise. During the dropping process, the dropping rate is controlled so that the reaction temperature is 10±2° C. and the pH value is maintained between 8.0-8.5. The dropwise addition is completed within 3-4h. After the dropwise addition, keep warm at 10-20°C for 4-5 hours. After the reaction solution is dissolved, take a sample and control it to detect the residue of 7-AVCA. When the residue of 7-AVCA is less than 1.0%, stop the reaction.
[0060] 5 kg of tert-butyl acetate and 4 kg of purified water were added to the system. Stand to separate layers, separate the water phase, add 4kg tert-butyl acetate to the water phase, and stir. Static layering, the tert-butyl acetate phase obtained and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com