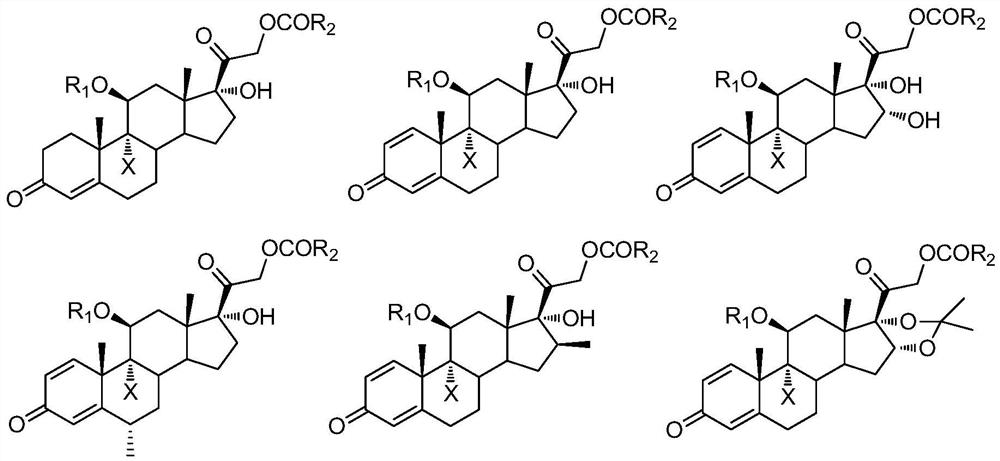
A kind of preparation method of 9-position dehalogenation of 9-halogenated steroid hormone compound
A technology of steroid hormones and compounds, which is applied in the field of medicine and chemical industry, can solve the problems of difficult treatment, large pollution of three wastes, and odor of mercapto fatty acid, etc., and achieve the effects of reducing pollution of three wastes, less reaction impurities, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
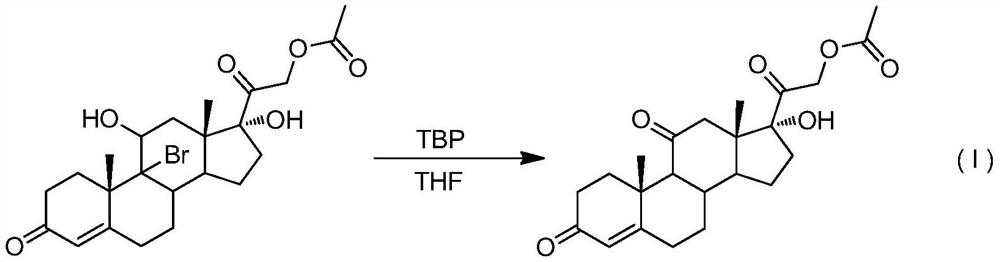
[0025] Add 48.2 g (0.10 mol, 1.0 equiv) of 9-bromo-acetic acid hydrocortisone in the reaction kettle under nitrogen protection conditions, 250 ml of tetrahydrofuran is heated and refluxed, and di-tert-butyl peroxide is slowly added dropwise (TBP) 7.3g (0.05mol, 0.5equiv), TLC detection to control the reaction end point, after the reaction is over, reduce the temperature of the reaction kettle, and distill under reduced pressure (distillation temperature does not exceed 40°C, vacuum degree is about -0.08MPa) to remove tetrahydrofuran About 150ml, the reaction system was charged into 1000ml of ice water, filtered, and dried to obtain 36.6g of white solid cortisone acetate, with a molar yield of 90.0% and an HPLC purity of >97.0%.
Embodiment 2
[0027]
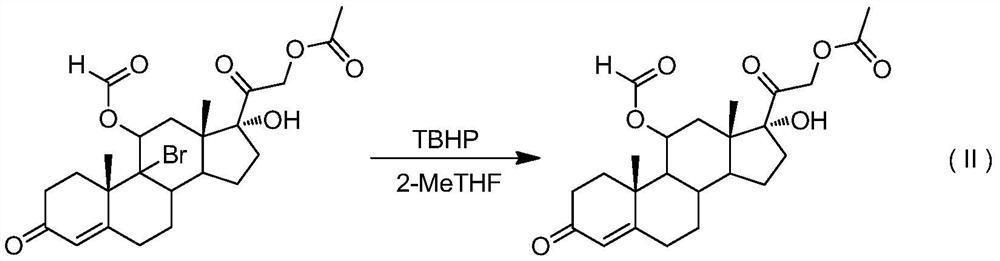
[0028] Add 9-bromo-11-formic acid ester-21-acetate hydrocortisone 51.0g (0.10mol, 1.0equiv) in the reaction flask under nitrogen protection conditions, 2-methyltetrahydrofuran 2500ml, Heat up to reflux, slowly add 18.0 g (0.20 mol, 2.0 equiv) of tert-butyl hydroperoxide (TBHP) dropwise, be careful not to let the water in tert-butyl hydroperoxide enter the reaction bottle, TLC detection controls the reaction end point, and wait for the reaction to end , reduce the temperature of the reaction kettle, and distill under reduced pressure (distillation temperature does not exceed 50°C, vacuum degree is about -0.08MPa) to remove about 2300ml of 2-methyltetrahydrofuran, cool the reaction system to 0°C, stir and crystallize for two hours, filter , and dried to obtain 39.8 g of white solid 11-formate-21-acetate hydrocortisone, the molar yield was 92.0%, and the HPLC purity was >98.5%.
Embodiment 3
[0030]
[0031] Add 48.0 g (0.10 mol, 1.0 equiv) of 9-bromo-prednisolone acetate in the formula (III) and 1000 ml of 3-methyltetrahydrofuran to the reaction flask under the condition of nitrogen protection, heat up and reflux, and slowly add peroxide Tert-butyl benzoate (TBPB) 38.8g (0.20mol, 2.0equiv), TLC detection to control the reaction end point, after the reaction is finished, reduce the temperature of the reactor, and distill under reduced pressure (distillation temperature does not exceed 50°C, vacuum degree is about -0.08 MPa) about 800ml of 3-methyltetrahydrofuran was removed, the reaction system was cooled to 0°C, stirred and crystallized for two hours, filtered, and dried to obtain 32.4g of prednisone acetate as a white solid, with a molar yield of 81.0%, HPLC purity>98.5 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com