Hyperbranched polyaminoester capable of emitting multicolor fluorescence and preparation method thereof
A technology of polyurethane and terminal hydroxyl hyperbranching, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of single chromatogram, low fluorescence intensity, low quantum yield, etc., and achieve simple synthesis process and high fluorescence intensity , good biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
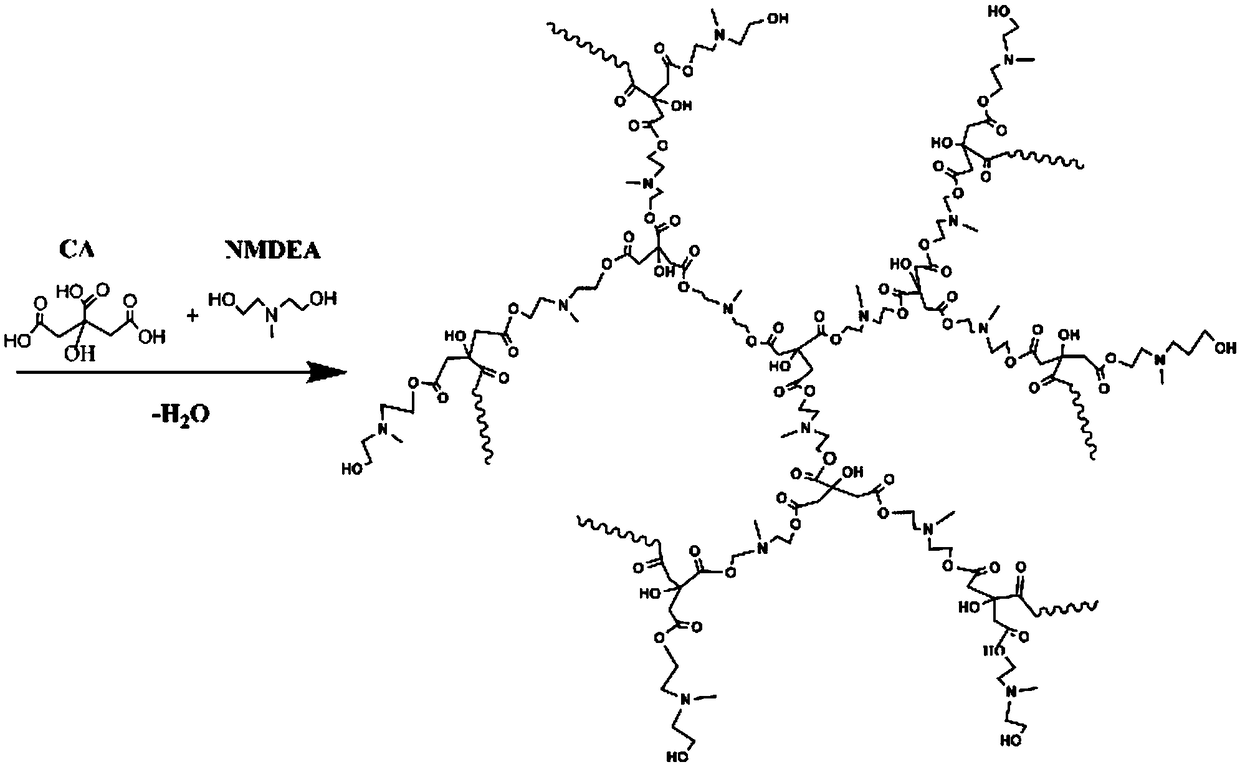
[0034] The preparation method of hydroxyl-terminated hyperbranched polyurethane: under the protection of nitrogen, citric acid and N-methyldiethanolamine are reacted at a molar ratio of 1:1.6, and the raw materials are dissolved at 120 ° C ~ 145 ° C, and the total mass of the reactants is added 0.5% p-toluenesulfonic acid, gradually increase the temperature to 160°C, and react for 4 hours. Then react for 4 hours under negative pressure environment, and the product is a blood-red viscous liquid. Next, the product was dissolved in water, precipitated by adding acetone, filtered, and vacuum-dried to obtain a hydroxyl-terminated hyperbranched polyurethane.
example 2
[0036] The preparation method of hydroxyl-terminated hyperbranched polyurethane: under the protection of nitrogen, citric acid and N-methyldiethanolamine are reacted at a molar ratio of 1:2.0, and the raw materials are dissolved at 120 ° C ~ 145 ° C, and the total mass of the reactants is added With 0.8% p-toluenesulfonic acid, gradually increase the temperature to 140°C, react for 6 hours, and then react for 6 hours under a negative pressure environment, and the product is a blood-red viscous liquid. Next, the product was dissolved in water, precipitated by adding acetone, filtered, and vacuum-dried to obtain a hydroxyl-terminated hyperbranched polyurethane.
example 3
[0038] The preparation method of hydroxyl-terminated hyperbranched polyurethane: under the protection of nitrogen, citric acid and N-methyldiethanolamine are reacted at a molar ratio of 1:2.2, and the raw materials are dissolved at 120 ° C ~ 145 ° C, and the total mass of the reactants is added With 0.1% p-toluenesulfonic acid, gradually increase the temperature to 160°C, react for 4 hours, and then react for 4 hours in a negative pressure environment, and the product is a blood-red viscous liquid. Next, the product was dissolved in water, precipitated by adding acetone, filtered, and vacuum-dried to obtain a hydroxyl-terminated hyperbranched polyurethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com