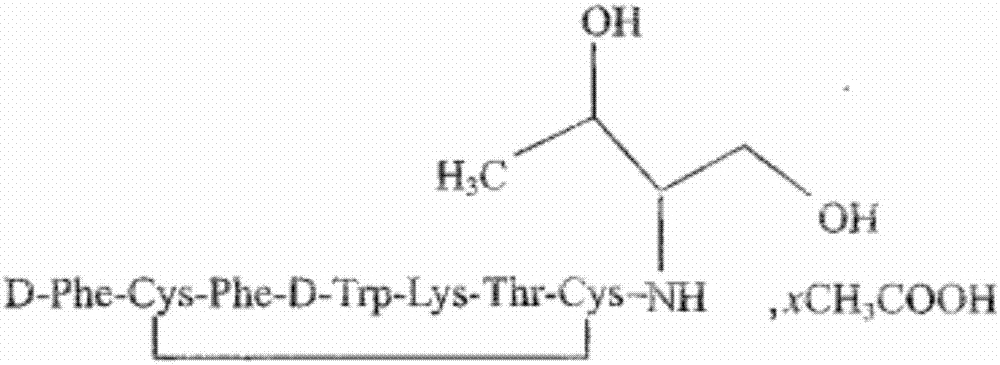
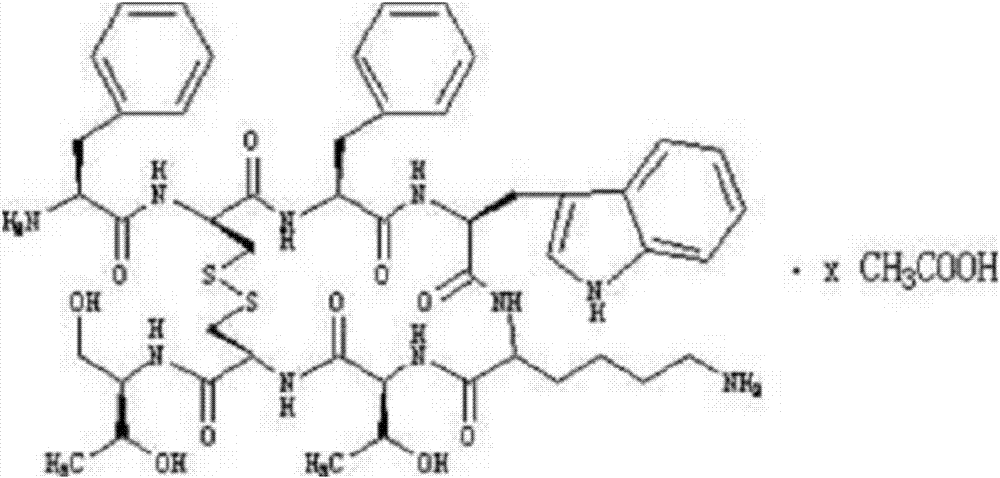
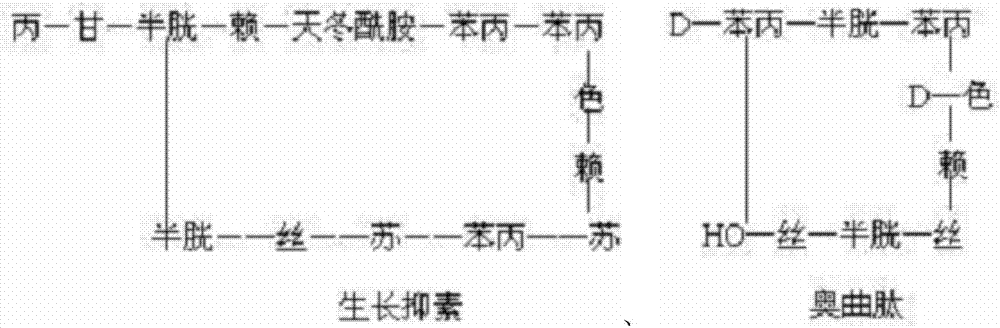
Preparation of octreotide acetate and octreotide acetate injection pharmaceutical composition
A technology of octreotide acetate and octreotide, which is applied in the field of medicine and can solve problems such as long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the preparation of octreotide acetate
[0084] 1. Synthetic peptide chain
[0085] (1) Preparation of Boc-Thr(tBu)-OCs
[0086] Take 30.3 g of Boc-Thr(tBu)-OH (FW: 275.3, 110 mmol), dissolve it in 250 ml of methanol and 250 ml of water, pH 2.5. Take another 24g of cesium carbonate (147mmol) and add 100ml of water to dissolve until clear, and slowly add the above-mentioned Boc-Thr(tBu)-OH solution under stirring, and carbon dioxide gas will be generated. The pH of the solution was 7.5. It was dissolved in water at 40-50°C and concentrated to dryness under reduced pressure to obtain 54.2 g of Boc-Thr(tBu)-OCs as a syrupy residue.
[0087] (2) Preparation of Boc-Thr(tBu)-O-resin
[0088] Get 100 grams of chloromethyl resin (100-200 mesh, 1.0mmol / g, 100mmol), soak in DMF for 30 minutes, make the resin fully swell, add step (1) gained syrupy residue 107g and DMF of 700ml, 50- React at 55°C for 48 hours.
[0089] After filtering, the resin was washed twice...
Embodiment 2
[0120] Embodiment 2: the preparation of octreotide acetate
[0121] 1. Synthetic peptide chain
[0122] With embodiment 1.
[0123] Two, disulfide bond ring formation
[0124] Dissolve 90g of the reduced octreotide crude product in 90L of water, slowly add 2M ammonia water dropwise to pH7.8 under stirring, and feed air into the solution at room temperature (the used air passes through saturated calcium hydroxide solution and 2M sulfuric acid solution in advance successively) ), so that the disulfide bonds undergo ring-forming reaction for about 24-36 hours, add glacial acetic acid to adjust the pH to 5.5, add activated carbon to stir for 30 minutes, and filter.
[0125] 3. Separation and purification
[0126] Gained whole filtrate is purified in batches through C18 column when disulfide bonds are formed into rings, mobile phase: 0.25mol / L potassium acetate: acetonitrile (7.2:2.8); Flow rate is: 800ml / min; Effluent, the detection wavelength is: 280nm; After the sample pea...
Embodiment 3
[0127] Embodiment 3: the preparation of octreotide acetate
[0128] 1. Synthetic peptide chain
[0129] With embodiment 1.
[0130] Two, disulfide bond ring formation
[0131] Dissolve 90g of the reduced octreotide crude product in 90L of water, slowly add 2M ammonia water dropwise to pH7.8 under stirring, and feed air into the solution at room temperature (the used air passes through 2M sulfuric acid solution and saturated calcium hydroxide solution successively in advance) ), so that the disulfide bonds undergo ring-forming reaction for about 24-36 hours, add glacial acetic acid to adjust the pH to 5.5, add activated carbon to stir for 30 minutes, and filter.
[0132] 3. Separation and purification
[0133] Gained whole filtrate is purified in batches through C18 column when disulfide bonds are formed into rings, mobile phase: 0.25mol / L potassium acetate: acetonitrile (7.2:2.8); Flow rate is: 800ml / min; Effluent, the detection wavelength is: 280nm; After the sample pea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com