Preparation method of 2-amino-4,6-dichloro-5-formamine pyrimidine
A technology of formamide and pyrimidine, which is applied in the field of pharmaceutical preparation, can solve problems such as unresolved inherent drawbacks, inability to recycle, difficult process control, etc., and achieve the effects of fast production cycle, short time consumption, and reduction of three wastes pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] In order to solve the above technical problems, the embodiment of the present invention provides a preparation method of 2-amino-4,6-dichloro-5-carboxamidopyrimidine, comprising the following steps:
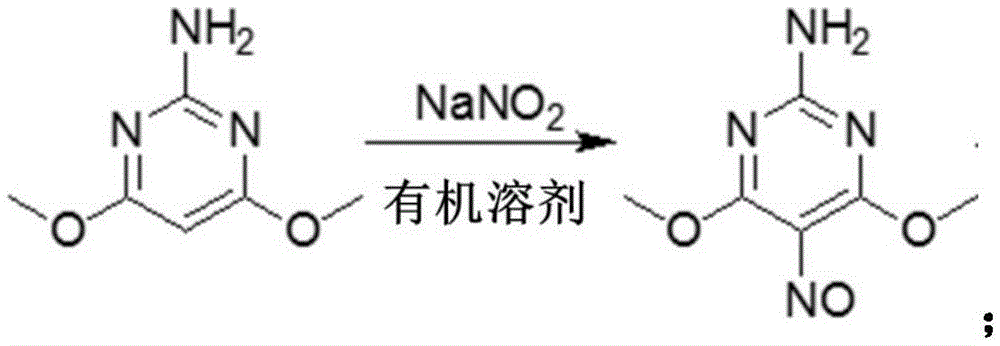
[0032] Step S1: In the case of using an organic solvent, ADMP undergoes nitrosation reaction with sodium nitrite to obtain 2-amino-4,6-dimethoxy-5-isonitrosopyrimidine:
[0033]
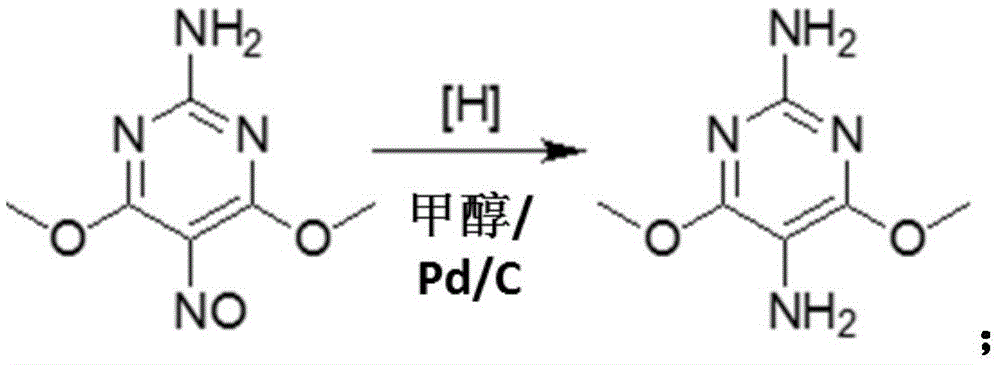
[0034] Step S2: In the case of using methanol and palladium on carbon as a catalyst, pass hydrogen gas into the 2-amino-4,6-dimethoxy-5-isonitrosopyrimidine obtained in step S1 to undergo a hydrogenation reduction reaction, and remove it by filtration palladium carbon catalyst, concentrated hydrochloric acid was added to the filtrate to obtain 2,5-diamino-4,6-dimethoxypyrimidine hydrochloride:
[0035]
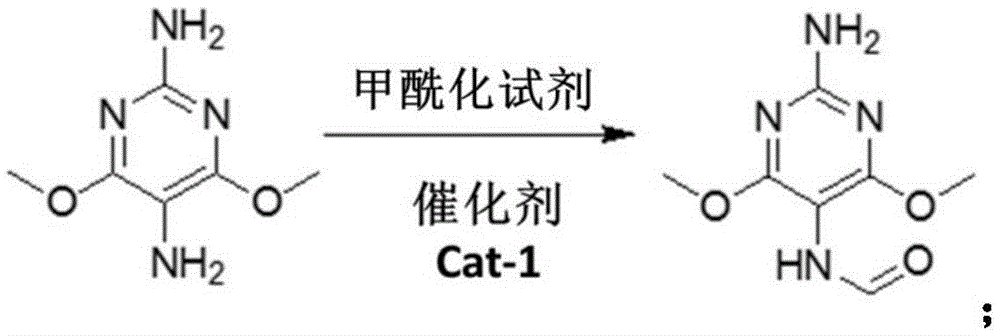
[0036] Step S3: In the case of using the catalyst Cat-1, the 2,5-diamino-4,6-dimethoxypyrimidine hydrochloride obtained in step S2 is subjected to a formylation reaction with a formylation ...
Embodiment 1
[0051] 1. Preparation of 2-amino-4,6-dimethoxy-5-isonitrosopyrimidine
[0052] Add 2-amino-4,6-dimethoxypyrimidine (31.0g, 0.2mol), methanol (200ml) and 40% aqueous sodium nitrite solution (38.0g, 0.22mol) successively in a 500ml three-necked flask, stir and mix Evenly, heat to 70-75°C, slowly add concentrated hydrochloric acid dropwise to system pH=2.5-3.0, consume concentrated hydrochloric acid (51.0g, 0.51mol) in total, continue to keep warm for 1 hour, take samples and monitor until the raw material content is <1% (HPLC- Area%), down to room temperature suction filtration to obtain a filter cake, the filter cake was washed twice with 50ml water, and dried at 50°C to obtain the crude product of 2-amino-4,6-dimethoxy-5-isonitrosopyrimidine 37.4g, content 94.5%, yield 96.1%, the product was confirmed by hydrogen nuclear magnetic resonance spectrum (1HNMR for short) and mass spectrometry (MS for short): 1HNMR (400Hz, DMSO-d6) 3.88(s, 6H), 7.85(s, 2H); MS (HPLC, ESI) 185 (M+H)...
Embodiment 2
[0064] 1. Preparation of 2-amino-4,6-dimethoxy-5-isonitrosopyrimidine
[0065] Add 2-amino-4,6-dimethoxypyrimidine (31.0g, 0.2mol), tetrahydrofuran (200ml) and 40% sodium nitrite aqueous solution (38.0g, 0.22mol) successively in a 500ml three-necked flask, stir and mix Evenly, heat to 60-65°C, slowly add concentrated hydrochloric acid dropwise to system pH=2.5-3.0, consume concentrated hydrochloric acid (51.0g, 0.51mol) in total, continue to keep warm for 3 hours, take samples and monitor until the raw material content is <1% (HPLC- Area%), down to room temperature suction filtration to obtain a filter cake, the filter cake was washed twice with 50ml water, and dried at 50°C to obtain the crude product of 2-amino-4,6-dimethoxy-5-isonitrosopyrimidine 35.6g, content 97.0%, yield 93.8%, the product was confirmed by 1HNMR and MS: 1HNMR (400Hz, DMSO-d6) 3.88 (s, 6H), 7.85 (s, 2H); MS (HPLC, ESI) 185 (M +H).
[0066] 2. Preparation of 2,5-diamino-4,6-dimethoxypyrimidine hydrochlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com