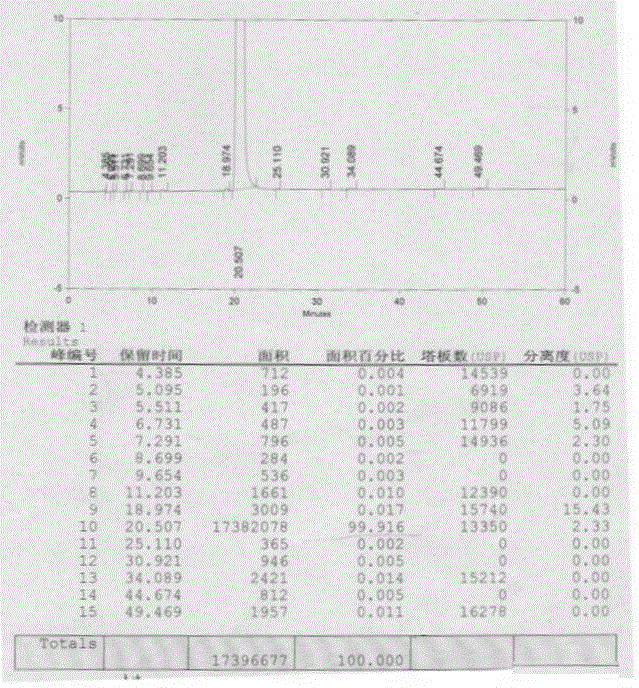
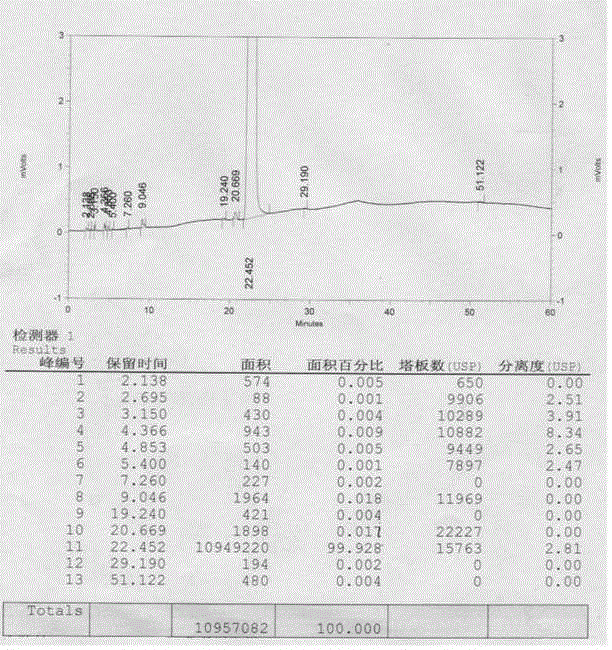
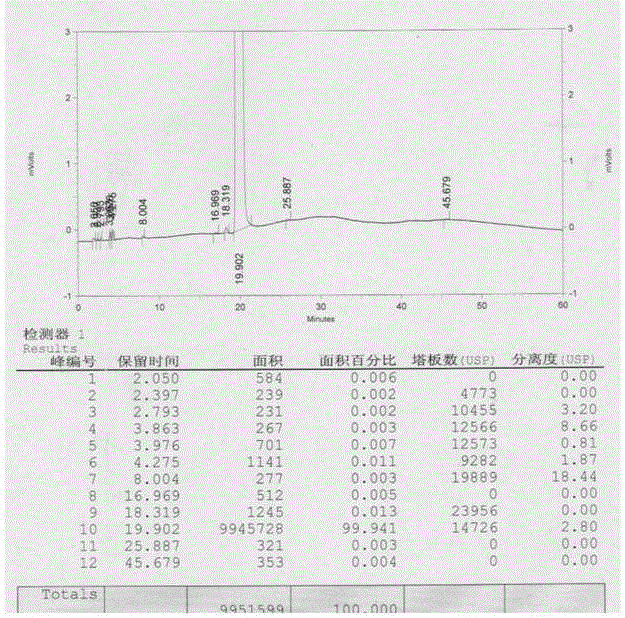
A kind of preparation method of n(2)-l-alanyl-l-glutamine
A technology of glutamine and alanyl, applied in the field of medicine, can solve the problems of high production cost, short reaction preparation process, rising market price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (1) Preparation of L-phthaloyl-alanyl chloride benzene solution:
[0068] In a 2-ton reaction kettle, dissolve 230kg of L-phthaloyl-alanine with 700kg of benzene, add 130kg of thionyl chloride, and react at 50°C for 3 hours. After the reaction is completed, the excess acylating reagent is evaporated to obtain 900kg of L- Phthaloyl-alanyl chloride benzene solution for subsequent use;
[0069] (2) Preparation of phthaloyl-L-alanyl-L-glutamic acid:
[0070] In a 2 ton reaction kettle, add 40kg of sodium hydroxide to 400kg of water and stir to dissolve. After dissolving, cool down to 5°C, add 147kg of L-glutamic acid, control the temperature of the reaction solution at 5-10°C, and add L-phthaloyl- 900kg of alanyl chloride benzene solution and 200kg of 50% sodium hydroxide solution, maintain the pH value of the reaction solution at 9.5-10.5; half an hour to complete the dropwise addition, and then keep the temperature for 2 hours. Adjust the acid with acetic acid to pH 2-3...
Embodiment 2
[0080] (1) Preparation of L-phthaloyl-alanyl chloride toluene solution:
[0081] Dissolve 230kg of L-phthaloyl-alanine in 2 tons of reactor with 700kg of toluene, cool down to below 10°C, add 106kg of solid phosgene, then add 10.6kg of catalytic amount of triethylamine, and slowly heat up within one hour to 60°C; heat-retaining reaction for 3 hours, after the reaction was completed, the excess acylating reagent was distilled under reduced pressure to obtain 900 kg of L-phthaloyl-alanyl chloride toluene solution for later use;
[0082] (2) Preparation of phthaloyl-L-alanyl-L-glutamic acid:
[0083] In a 2 ton reaction kettle, add 40kg of sodium hydroxide to 400kg of water and stir to dissolve. After dissolving, cool down to 5°C, add 147kg of L-glutamic acid, control the temperature of the reaction solution at 5-10°C, and add L-phthaloyl- 900kg of alanyl chloride benzene solution and 550kg of 40% potassium carbonate solution, control the reaction temperature at 5-10°C, and main...
Embodiment 3
[0093] (1) Preparation of L-phthaloyl-alanyl chloride toluene solution:
[0094] In a 2-ton reaction kettle, dissolve 230kg of L-phthaloyl-alanine with 700kg of toluene, add 130kg of thionyl chloride, heat up to 50°C within one hour and react for 3 hours. After the reaction is completed, the excess acylating reagent is evaporated. , obtain 900kgL-phthaloyl-alanyl chloride toluene solution for subsequent use;
[0095] (2) Preparation of phthaloyl-L-alanyl-L-glutamic acid:
[0096] In a 2 ton reaction kettle, add 40kg of sodium hydroxide to 400kg of water and stir to dissolve. After dissolving, cool down to 5°C, add 147kg of L-glutamic acid, control the temperature of the reaction solution at 5-10°C, and add L-phthaloyl- 900kg of alanyl chloride toluene solution and 760kg of 25% sodium carbonate solution were used to maintain the pH value of the reaction solution at 9.5-10.5; the dropwise addition was completed in about half an hour, and then kept for 2 hours for reaction. Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com