Method for preparing 2-benzyl pyridine compound
A technology for benzylpyridine and compounds, applied in the field of preparing 2-benzylpyridine compounds, which can solve the problems of high molecular weight, poor atom economy, and low yield of synthetic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
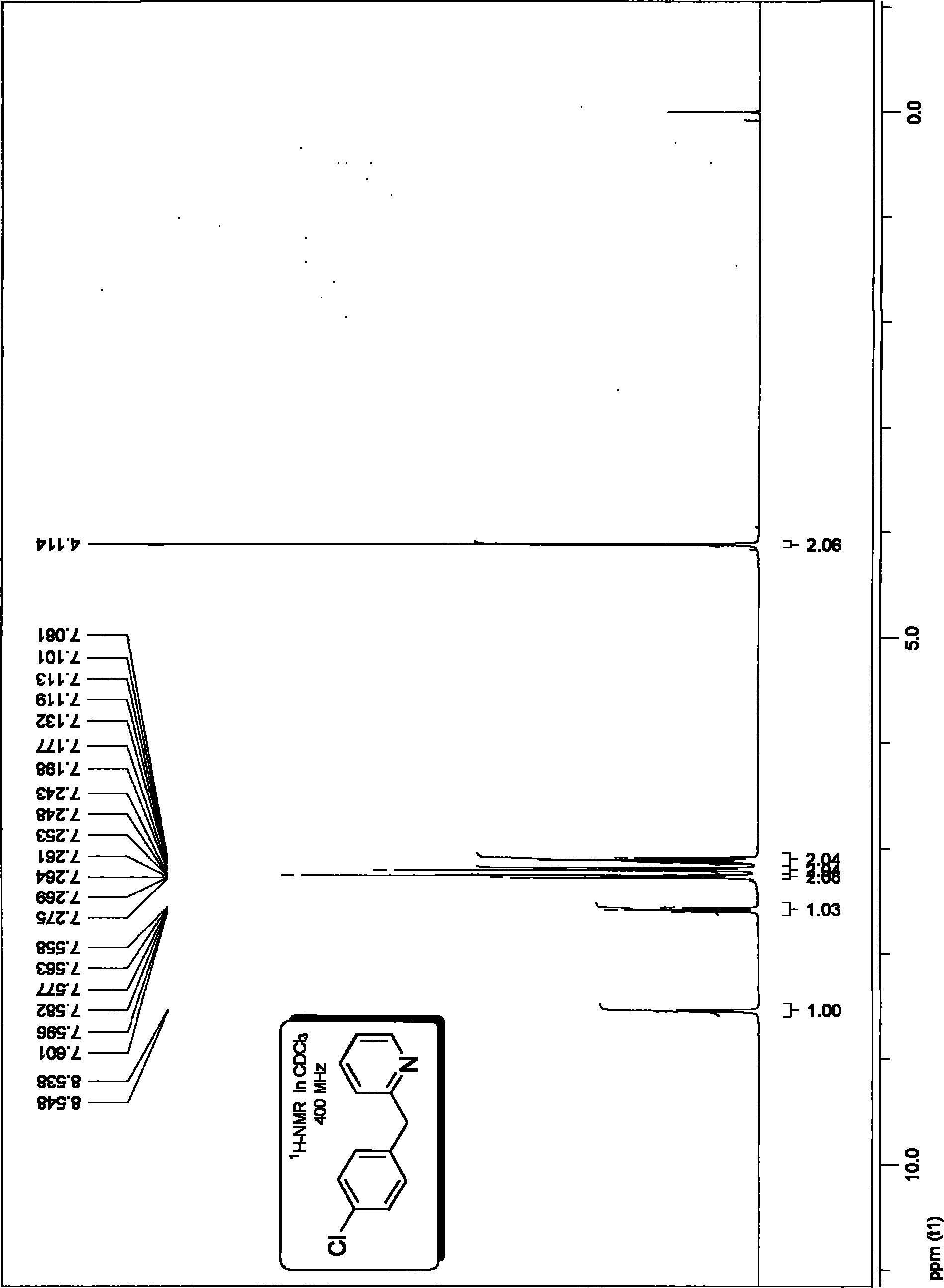
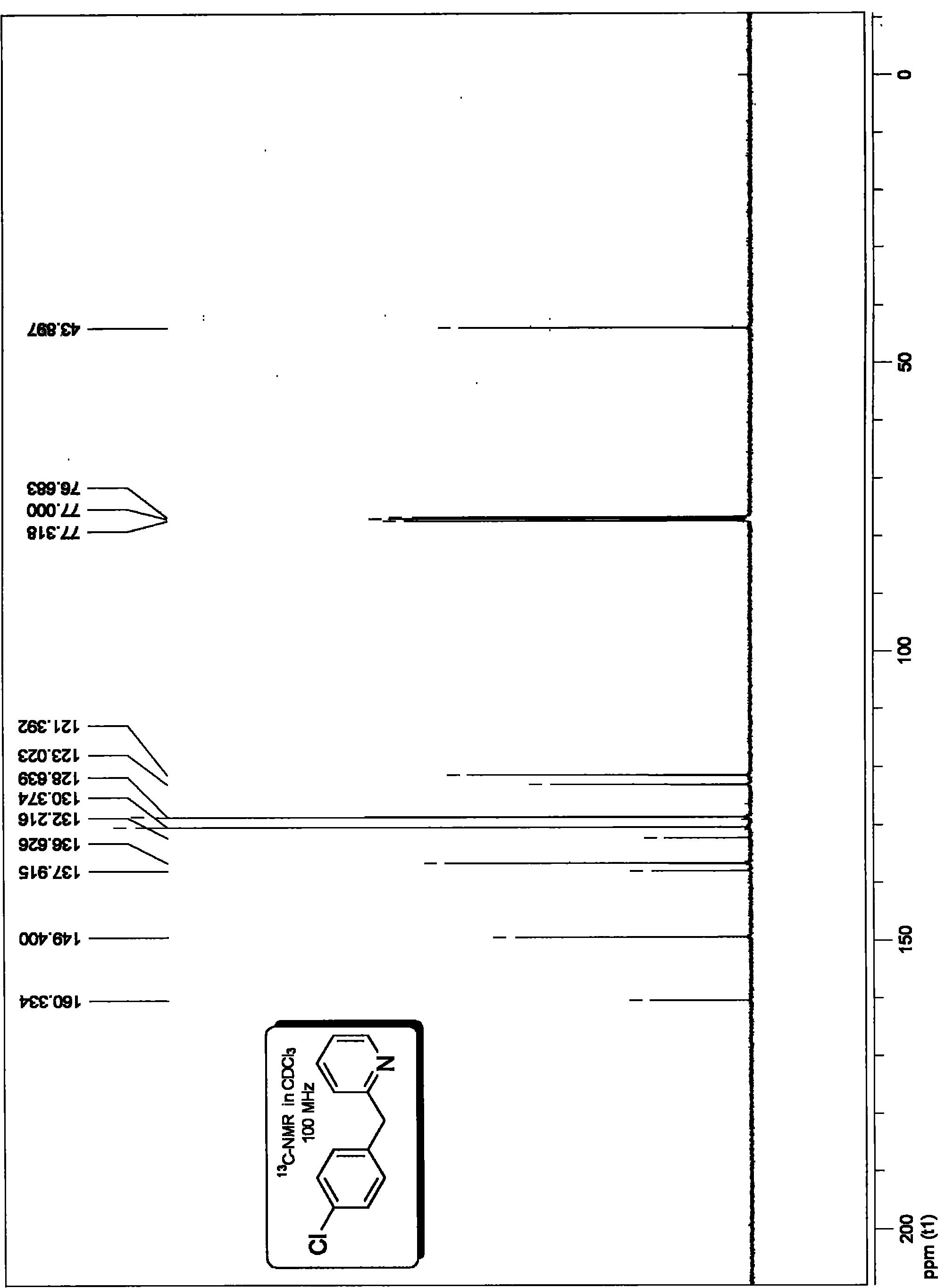
[0066] Embodiment 1, preparation p-chloro 2-benzylpyridine:
[0067] Reaction formula:
[0068]
[0069] The specific method is as follows: in the vacuum reactor, add 1.2 millimoles of 2-pyridine acetic acid potassium salt, and the molar dosage is 0.005 millimoles of three (dibenzylidene acetone) dipalladium catalysts and electrophilic substrate molar dosage 0.5%. 0.015 millimoles of Xant-Phos phosphine ligand with a molar dosage of 1.5%, vacuumize, pass high-purity argon, replace three times, and add 1 millimole of electrophilic substrate p-chlorobromobenzene and solvent under the protection of argon flow Diethylene glycol dimethyl ether (the amount of solvent is added according to 0.2 milliliters of solvent per millimole of electrophilic substrate), placed at 150 ° C, heated and stirred for 24 hours, according to the following method for the reaction system after the completion of the decarboxylation coupling reaction Purification treatment to obtain the target product p...
Embodiment 2
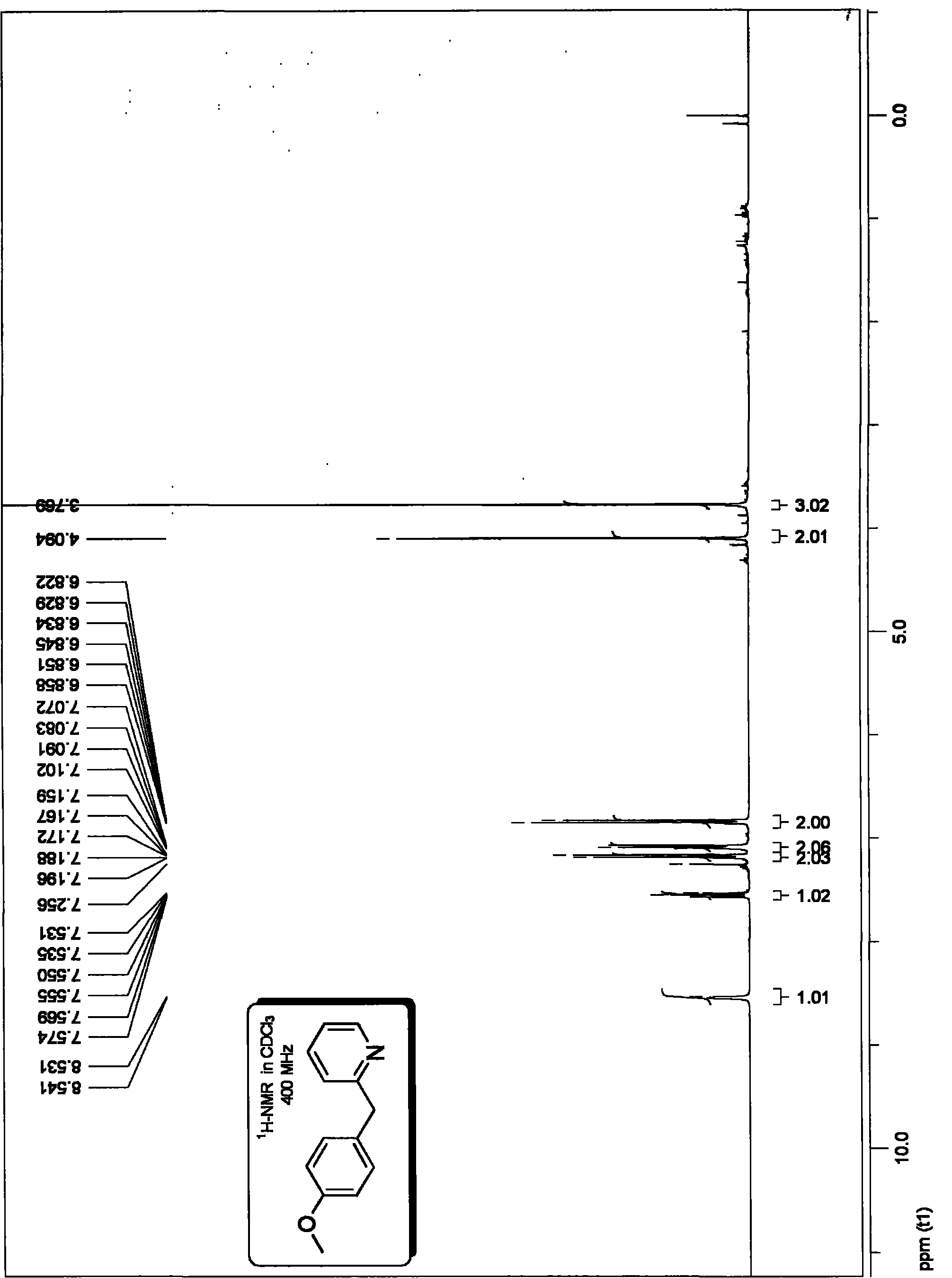
[0071] Embodiment 2, preparation 4-methoxyphenyl-2-pyridyl methane:
[0072] Reaction formula:
[0073]
[0074] The specific method is as follows: in the vacuum reactor, add 1.2 millimoles of 2-pyridine acetic acid potassium salt, 1 millimole of electrophilic substrate p-methoxy bromobenzene, and the molar dosage is 0.5% tris( Dibenzylidene acetone) Dipalladium catalyst 0.005 millimoles and molar dosage are 0.015 millimoles of Xant-Phos phosphine ligand of electrophilic substrate molar dosage 1.5%, vacuumize, logical high-purity argon, displacement three times, in argon flow Add the solvent 1,3,5-trimethylbenzene (mesitylene) under the protection of protection (the amount of solvent is added according to 0.2 ml per millimole of the electrophilic substrate), place it at 150 ° C, heat and stir for 24 hours, and the reaction system after the reaction is completed The mixture was filtered, and the reaction solution was directly chromatographed by fast silica gel column chroma...
Embodiment 3
[0076] Embodiment 3, preparation 2-pyridyl-3-pyridyl methane:
[0077] Reaction formula:
[0078]
[0079] The specific method is as follows: add 1.2 millimoles of 2-pyridine acetic acid potassium salt in the vacuum reactor, and the molar dosage is 0.005 millimoles and molar dosage of tris(dibenzylideneacetone) dipalladium catalyst of 0.5% electrophilic substrate molar dosage 0.015 millimoles of Xant-Phos phosphine ligand with an amount of 1.5% for the electrophilic substrate, vacuumize, pass high-purity argon, replace three times, and add 1 millimole of the electrophilic substrate 3-bromo under the protection of the argon flow Pyridine and the solvent diethylene glycol dimethyl ether (the amount of solvent is added according to 0.2 milliliters of solvent per millimole of electrophilic substrate), placed at 150 ° C, heated and stirred for 24 hours, according to the following method for the completion of the decarboxylation coupling reaction The reaction system is purified ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com