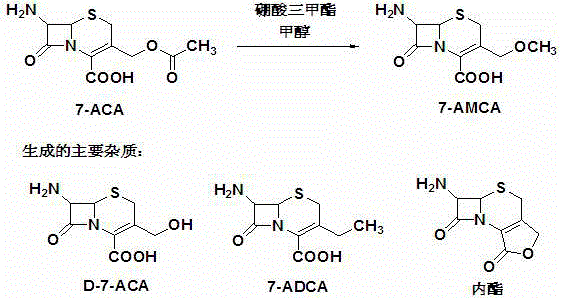
Preparation method of 7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid
A technology of aminocephalosporanic acid and methoxymethyl, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as difficulties in the preparation of dicarbonium tetrafluoroborate, unsuitability for industrial production, and difficulty in product separation, and achieve low production costs , shorten the reaction time, the effect of easy to obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Under nitrogen protection, add 300ml dimethyl sulfoxide to the reaction flask, add 60g (0.22mol) 7-ACA, control the temperature at 30-35°C, add 0.8g of imidazole, add hexamethyldisilazane 17.75g (0.11 mol), stirred for 60 minutes after adding, vacuumed for 30 minutes to remove the generated ammonia, then cooled to 0-5°C, added 23.5g (0.23mol) of trimethyl borate, 8.3g (0.26mol) of methanol, and then added 317.1 g (3.3mol) methanesulfonic acid, slowly add dropwise a mixture of 23.5g (0.23mol) trimethyl borate and 8.3g (0.26mol) methanol, the feeding time is 60min; after the addition, the temperature is controlled at 0-5°C , react for 2-3 hours; take a sample after 2 hours, measure 7-ACA residue, HPLC peak area normalization method ≤0.5%, cool the system to -10°C; cool 90ml of cold water (0-3°C) drop into the system After the dropwise addition is completed, control the temperature at 0-5°C, stir for 30 minutes, filter, and transfer the filtrate to a crystallization bottle...
Embodiment 2
[0035]Under nitrogen protection, add 300ml of dioxane to the reaction flask, add 60g (0.22mol) 7-ACA, control the temperature at 30-35°C, add 0.8g of imidazole, add 17.75g (0.11mol) of hexamethyldisilazane ), stirred for 60 minutes after adding, vacuumed for 30 minutes to remove the generated ammonia gas, then cooled to 0-5°C, added 23.5g (0.23mol) trimethyl borate, 8.3g (0.26mol) methanol, and then added 317.1g (3.3mol) methanesulfonic acid, slowly add dropwise a mixture of 23.5g (0.23mol) trimethyl borate and 8.3g (0.26mol) methanol, the addition time is 60min; after the addition, the temperature is controlled at 0-5°C, React for 2 to 3 hours; take a sample after 2 hours, measure the 7-ACA residue, and the HPLC peak area normalization method is ≤0.5%; cool the system to -10°C; drop 90ml of cold water (0-5°C) into the system, After the dropwise addition is completed, control the temperature at 0-5°C and stir for 30 minutes; filter and transfer the filtrate to a crystallizatio...
Embodiment 3
[0037] Under nitrogen protection, add 300ml dimethyl sulfoxide to the reaction flask, add 60g (0.22mol) 7-ACA, control the temperature at 30-35°C, add 0.8g imidazole, add N, O-bistrimethylsilyl ethyl Amide 22.38g (0.11mol), add and stir for 60 minutes, then cool down to 0-5°C, add 23.5g (0.23mol) trimethyl borate, 8.3g (0.26mol) methanol, then add 317.1g (3.3mol) Methanesulfonic acid, slowly drop a mixture of 23.5g (0.23mol) trimethyl borate and 8.3g (0.26mol) methanol, the addition time is 60min; 3 hours; take a sample after 2 hours, measure 7-ACA residue, HPLC peak area normalization method ≤0.5%; cool down the system to -10°C; drop 90ml of cold water (0-5°C) into the system, and the addition is complete , control the temperature at 0-5°C, stir for 30min; filter, and transfer the filtrate to a crystallization bottle; Add ammonia solution to the system, adjust pH=3.0-3.5; grow crystals at 0-5°C for 60 minutes; wash with 100ml of cold water (0-5°C), wash with 100ml of cold me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com