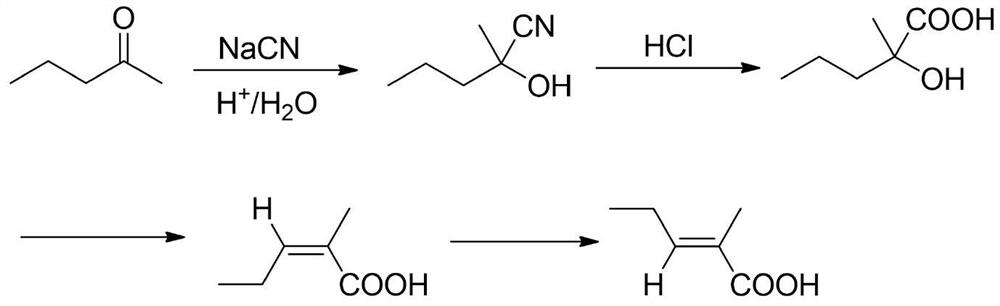
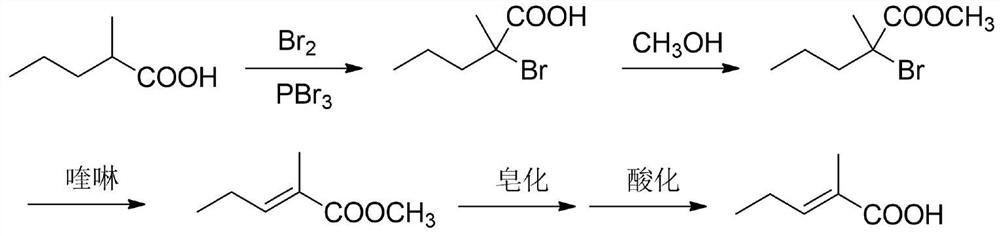
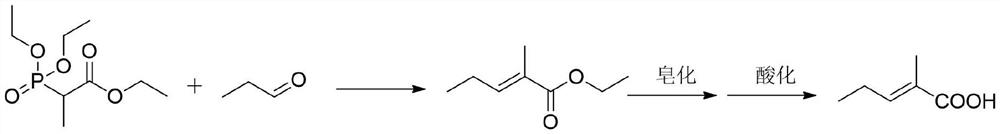
Synthesis method of trans-2-methyl-2-pentenoic acid
A synthesis method, technology of pentenoic acid, applied in the direction of carbon monoxide reaction to prepare carboxylic acid, organic chemical method, chemical instrument and method, etc., can solve the problems of insignificant industrial application benefits, many reaction steps, low yield, etc., and achieve atomic Good utilization rate, low production cost, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-25
[0063] Add catalyst 1 (rhodium salt and ligand) into the autoclave, add a solvent and stir to coordinate and dissolve the rhodium salt and ligand, then add piperylene and water. Close the reaction kettle, first replace the air in the kettle with nitrogen three times, then replace it with carbon monoxide three times, raise the temperature to the set temperature, and use carbon monoxide to replenish the pressure to the set pressure. The reaction is started, and the end point of the reaction is detected by the gas phase, and then the reaction liquid is moved to a rectification tower for vacuum distillation and separation to obtain the cis and trans isomers of 2-methyl-3-pentenoic acid. The specific experimental results are shown in Table 1 below.
[0064] Table 1 Experimental results of step (1)
[0065]
[0066]
[0067] According to the results in the above table, we can draw the following conclusions: comparative examples 1-4, the rhodium salt is preferably Rh 2 (CO) ...
Embodiment 26-36
[0069] Add a certain amount of catalyst 2 (zinc chloride / acetic acid solution) to the cis-trans isomers of 2-methyl-3-pentenoic acid obtained in step (1), and heat up to a set temperature to carry out isomerization reaction. The end point of the reaction is detected by the gas phase. After the reaction is completed, the reaction is washed twice with distilled water, and the upper oil phase is obtained by liquid separation. Then the reaction liquid is moved to a rectification tower for vacuum distillation and separation to obtain trans-strastracaric acid. The specific experimental results are shown in Table 2 below.
[0070] Table 2 Experimental results of step (2)
[0071]
[0072] According to the result in the above table, we can draw the following conclusions: the charging capacity of zinc chloride / acetic acid catalyst is preferably 8-13% (with the mass ratio of cis and trans isomers), and the chlorination in zinc chloride / acetic acid solution The mass concentration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com