Preparation method of PIM kinase inhibitor and intermediate of PIM kinase inhibitor
A volume and catalyst technology, applied in the direction of organic chemistry, etc., can solve the problems of easy decomposition, unstable raw material compound B1, unsuitable for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
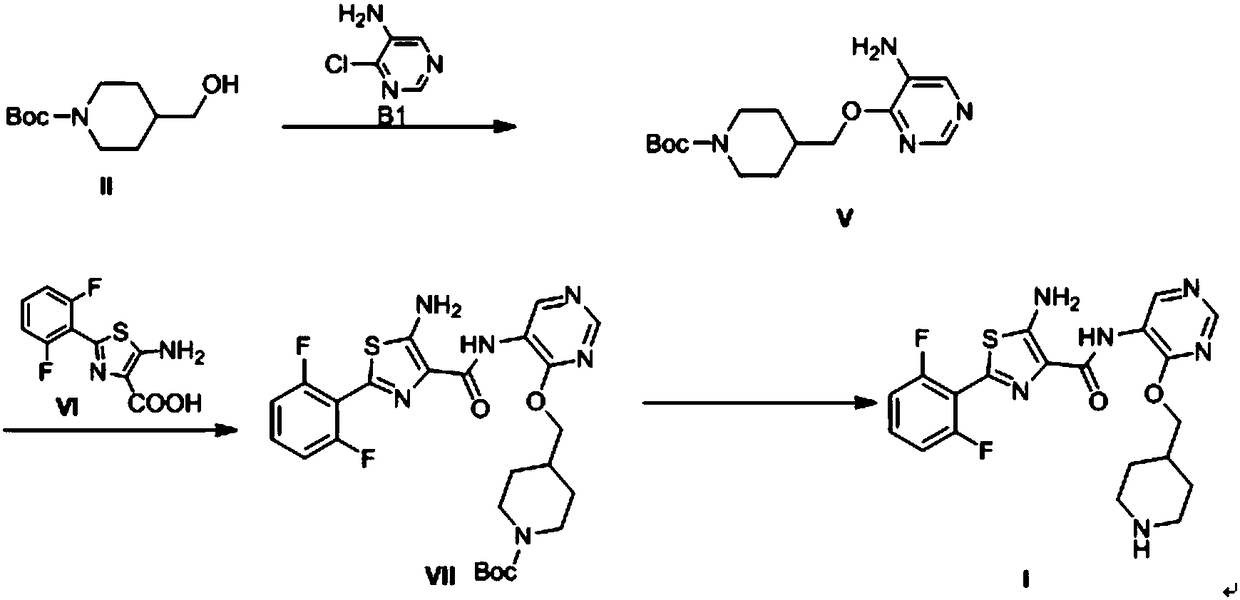
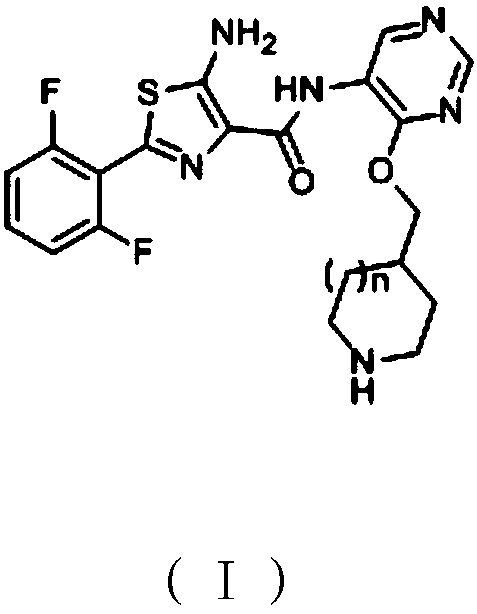
[0107] Embodiment 1: the preparation of the compound shown in formula I of the present invention
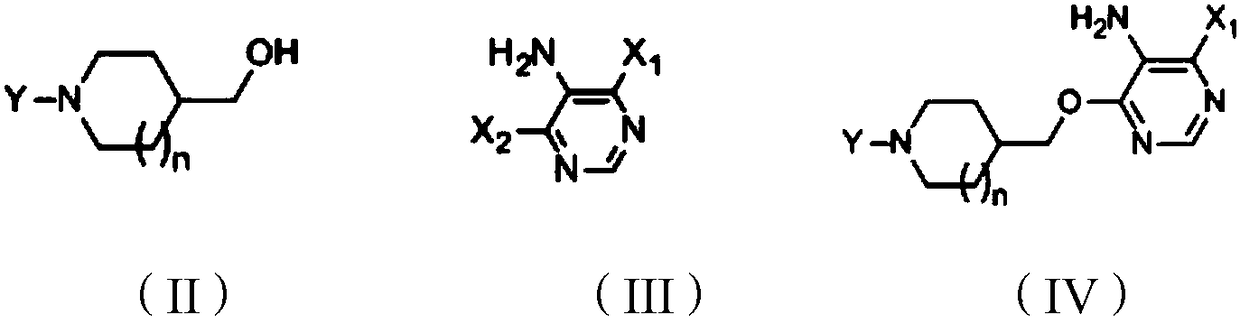
[0108] (1) Preparation of compound shown in formula IV
[0109] 4-(Hydroxymethyl)piperidine-1-carboxylic acid tert-butyl ester (compound shown in formula II) (2.15g, 10mmol), tetrahydrofuran (20ml) and sodium hydrogen (0.48g, 20mmol) were added to the reaction flask , stirred for 2h, added 4,6-dichloro-5-aminopyrimidine (compound represented by formula III) (1.49g, 9.1mmol), raised the temperature to 50-60°C for 2-3h, cooled to 0-5°C in an ice bath , drop 5ml of methanol, then use acetic acid to adjust the pH to 6-7, reduce the pressure to -0.09Mpa at 40°C, evaporate the above solvent to dryness, add 20ml of ethyl acetate and 20ml of water to the obtained residue, stir for 5 minutes, and let it stand Place and separate the layers, add 5g of anhydrous sodium sulfate to the obtained ethyl acetate layer and dry for 1h, filter, reduce the pressure to -0.09Mpa at 50°C and evaporate ...
Embodiment 2
[0119] Embodiment 2: the preparation of the compound shown in formula I of the present invention
[0120] (1) Preparation of compound shown in formula IV
[0121] Add tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate (compound represented by formula II) (2.15g, 10mmol) and 20ml of acetonitrile into the reaction flask, stir for 3h, then cool down with dry ice ethanol solution To below -20°C, slowly add 15ml of 2mol / L n-butyl lithium and tetrahydrofuran solution (30mmol) dropwise, the temperature does not exceed 0°C during the dropwise addition, stir at 0-5°C for 2h after the dropwise addition, add 4,6 - Dichloro-5-aminopyrimidine (compound shown in formula III) (3.28g, 20mmol), heat up to 30-40°C for 2-3h, cool down to 0-5°C in an ice bath, add 5ml of methanol dropwise, and then use Adjust the pH to 6-7 with acetic acid, and evaporate the solvent under reduced pressure to -0.09Mpa at 40°C. Add 20ml of ethyl acetate and 20ml of water to the obtained residue, stir for 5 min...
Embodiment 3
[0125] Embodiment 3: the preparation of the compound shown in formula I of the present invention
[0126] (1) Preparation of compound shown in formula IV
[0127]Add 4-(hydroxymethyl)piperidine-1-carboxylic acid tert-butyl ester (compound shown in formula II) (2.15g, 10mmol) and 20ml of 1,4-dioxane into the reaction flask, dry ice ethanol Cool the solution below -20°C, slowly add 2.5ml of 2mol / L n-butyllithium and tetrahydrofuran solution (5mmol) dropwise, the temperature does not exceed 0°C during the dropwise addition, stir at 0-5°C for 2h after the dropwise addition, add 0.82 g of 4,6-dichloro-5-aminopyrimidine (compound shown in formula III) (5mmol), heated up to 70-80°C for 2h, cooled to 0-5°C in an ice bath, dropped into 5ml of methanol, and then Adjust the pH to 6-7 with acetic acid, evaporate the solvent under reduced pressure to -0.09Mpa at 40°C, add 20ml of ethyl acetate and 20ml of water to the obtained residue, stir for 5 minutes, let stand, and separate the liq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com