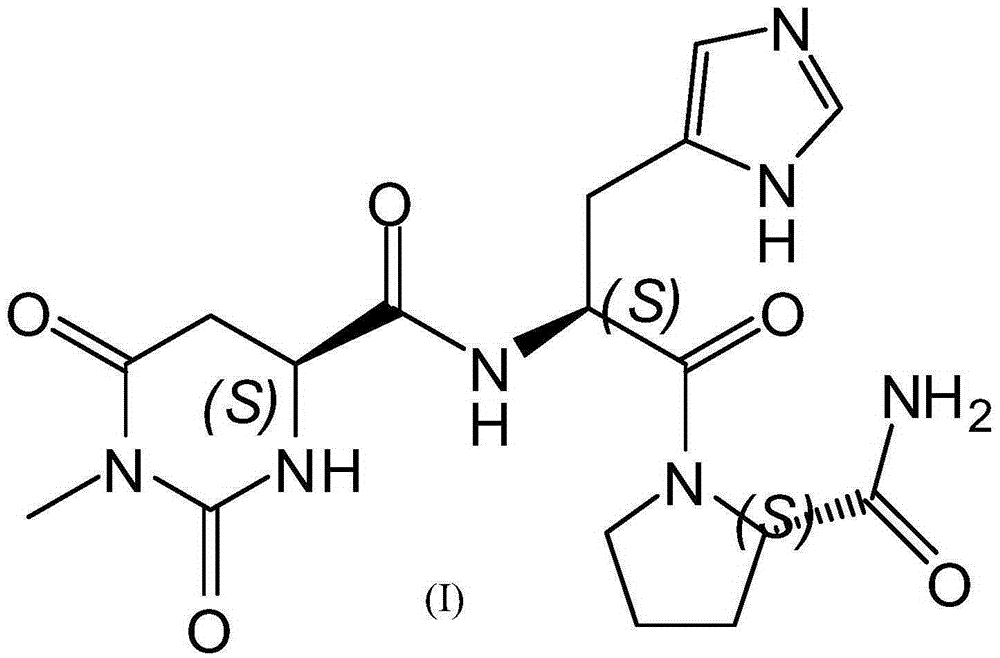
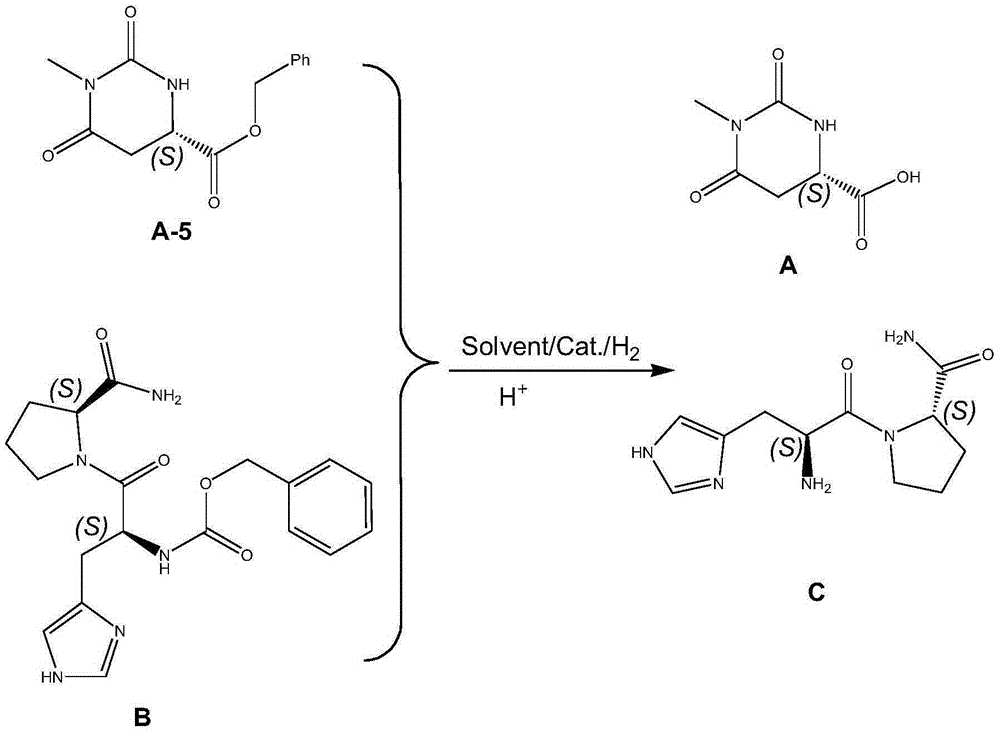
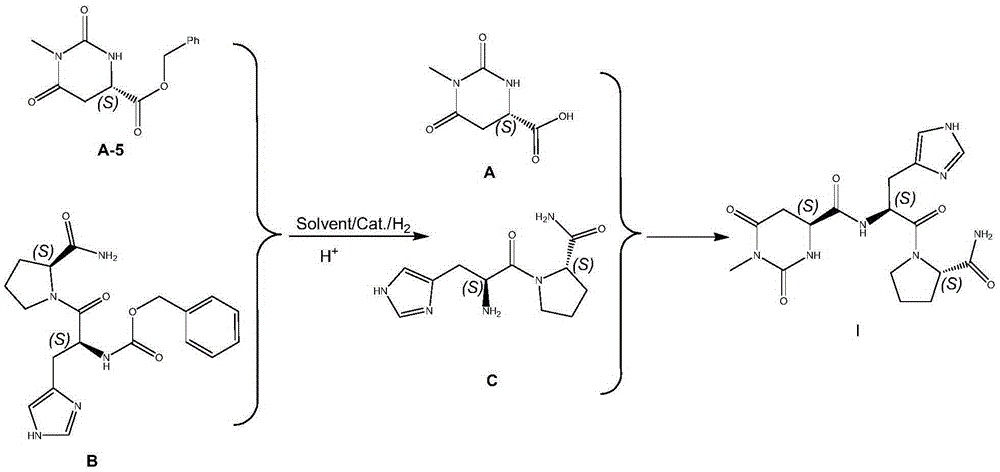
Preparing method for taltirelin and midbody of taltirelin
A tatirelin and intermediate technology, applied in the field of medicinal chemistry, can solve the problems of cumbersome operation, harsh reaction conditions, low yield, etc., and achieves the improvement of reaction yield, simple reaction conditions and high methylation selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) preparation formula N-2 compound is L-aspartic acid-4-carboxamide:
[0049] Mix the compound of formula N-1, that is, L-aspartic acid-4-methyl ester, with 10% methylamine-methanol solution at a mass ratio of 1:5, in a closed container at 25°C, The reaction occurred under the condition of 0psi, and the reaction time was 12 hours. After the reaction was completed, it was cooled to room temperature and concentrated under reduced pressure to obtain a light yellow semi-solid product, that is, L-aspartic acid-4-carboxamide, which was directly used for next step reaction;
[0050] (2) preparation formula N-3 compound is L-aspartic acid benzyl ester-4-formamide:
[0051] The compound of formula N-2, that is, L-aspartic acid-4-formamide and sodium hydroxide solution is salified, and the mol ratio of L-aspartic acid-4-formamide and sodium hydroxide is 1:1, and then Add dichloromethane, add tributylmethylammonium chloride and benzyl bromide to react at -5°C, the molar ratio ...
Embodiment 2
[0059] (1) preparation formula N-2 compound is L-aspartic acid-4-carboxamide:
[0060] Mix the compound of formula N-1, that is, the hydrochloride salt of L-aspartic acid-4-methyl ester, with the methylamine methanol solution with a mass concentration of 40% at a mass ratio of 1:20, in a closed container at 100 ° C, 50 psi The reaction occurred under the following conditions, and the reaction time was 2 hours. After the reaction was completed, it was lowered to room temperature, and concentrated under reduced pressure to obtain a light yellow semi-solid product, that is, L-aspartic acid-4-carboxamide, which was directly used in the next step without purification. one step reaction;
[0061] (2) preparation formula N-3 compound is L-aspartic acid benzyl ester-4-formamide:
[0062] The compound of formula N-2, that is, L-aspartic acid-4-formamide and sodium hydroxide solution is salified, and the mol ratio of L-aspartic acid-4-formamide and sodium hydroxide is 1:1.5, and then ...
Embodiment 3
[0070] (1) preparation formula N-2 compound is L-aspartic acid-4-carboxamide:
[0071] Mix the compound of formula N-1, that is, L-aspartic acid-4-methyl ester, with 40% methylamine methanol solution at a mass ratio of 1:8, and react in a closed container at 45°C and 25psi , the reaction time was 6 hours. After the reaction was completed, it was lowered to room temperature and concentrated under reduced pressure to obtain a light yellow semi-solid product, i.e. L-aspartic acid-4-carboxamide, which was directly used in the next step without purification;
[0072] (2) preparation formula N-3 compound is L-aspartic acid benzyl ester-4-formamide:
[0073] The compound of formula N-2, that is, L-aspartic acid-4-formamide and sodium hydroxide solution is salified, and the mol ratio of L-aspartic acid-4-formamide and sodium hydroxide is 1:1.2, and then Add ethyl acetate, add tetrabutylammonium chloride and benzyl bromide to react at 25°C, the molar ratio of L-aspartic acid-4-carboxa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com