Patents
Literature
56 results about "Blonanserin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
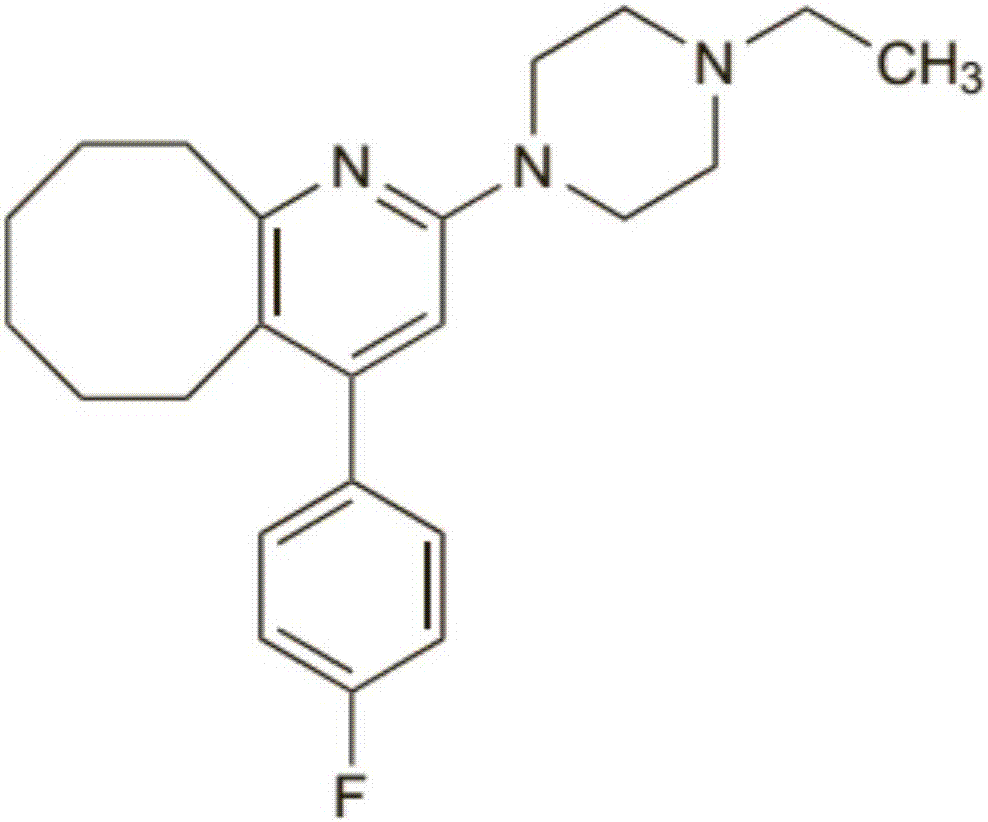
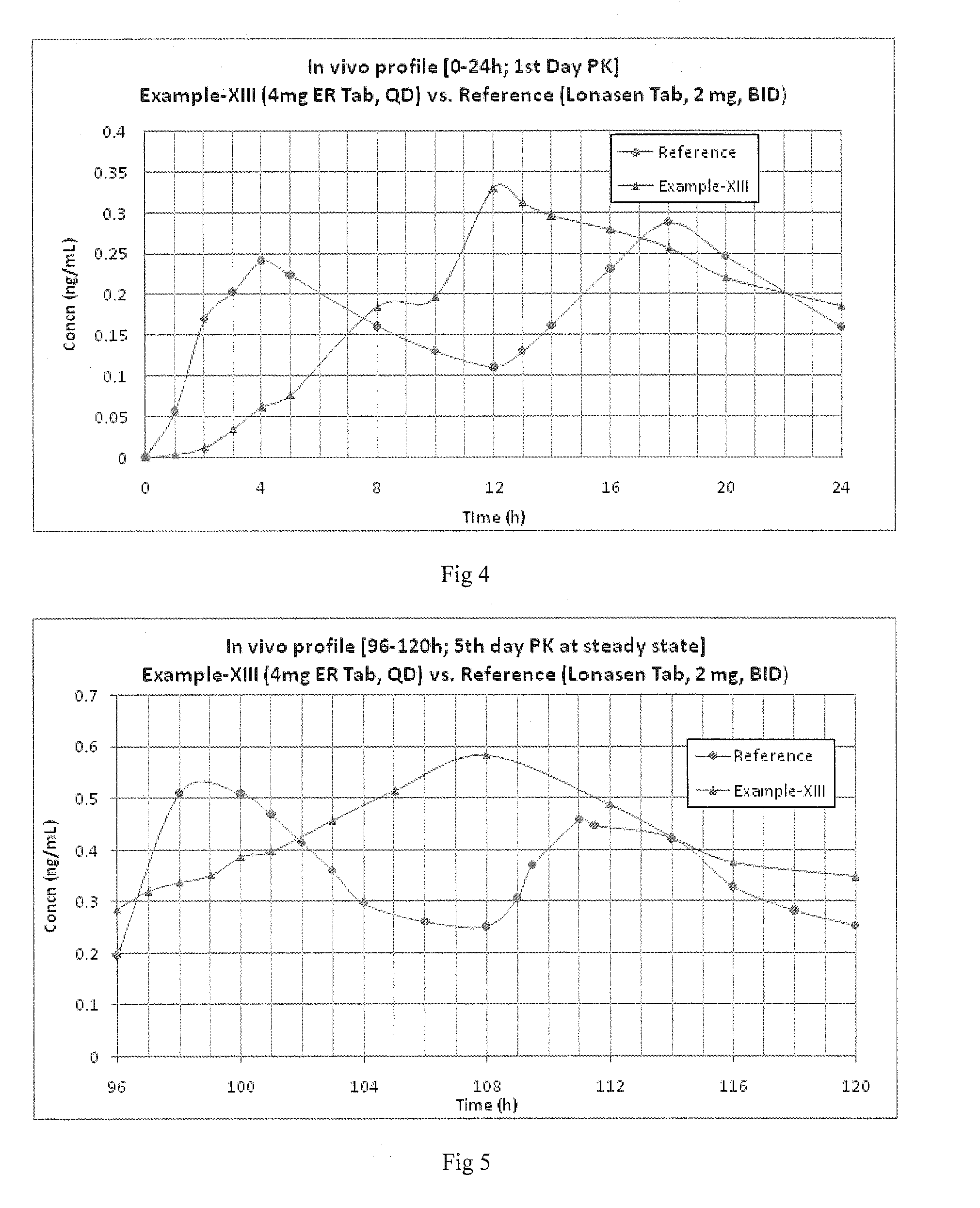
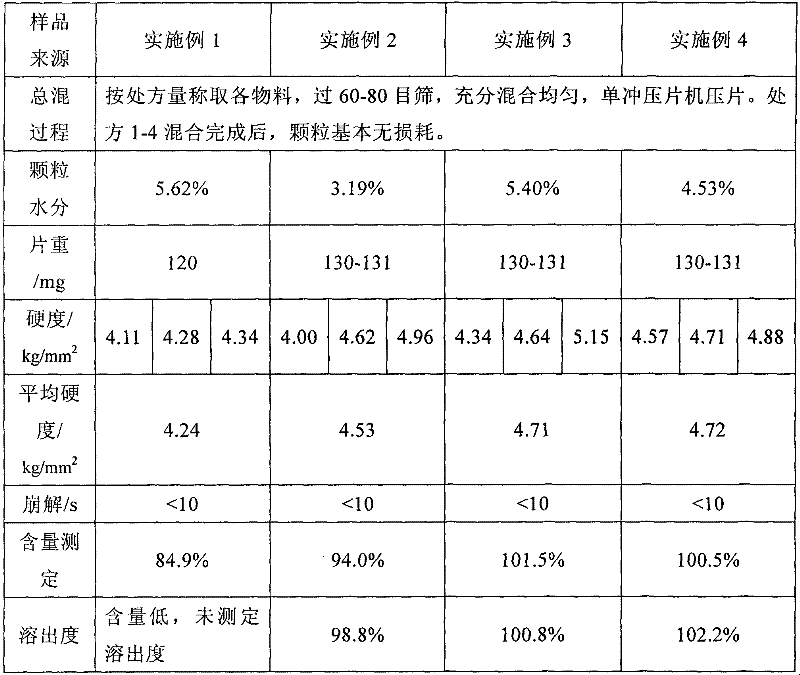
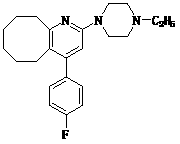
Blonanserin, sold under the brand name Lonasen, is a relatively new atypical antipsychotic (approved by PMDA in January 2008) commercialized by Dainippon Sumitomo Pharma in Japan and Korea for the treatment of schizophrenia. Relative to many other antipsychotics, blonanserin has an improved tolerability profile, lacking side effects such as extrapyramidal symptoms, excessive sedation, or hypotension. As with many second-generation (atypical) antipsychotics it is significantly more efficacious in the treatment of the negative symptoms of schizophrenia compared to first-generation (typical) antipsychotics such as haloperidol.
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
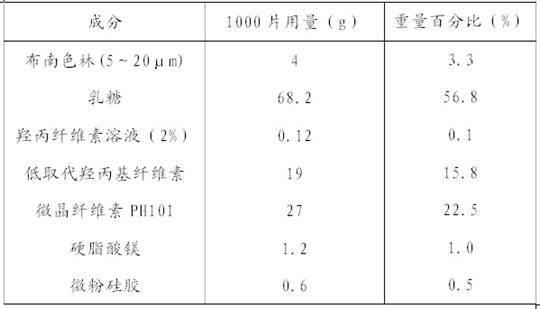
Blonanserin-containing medicinal composition and preparation method thereof
InactiveCN102078321ASolve solubilitySuitable for industrial productionOrganic active ingredientsNervous disorderBlonanserinAdhesive
The invention discloses a blonanserin-containing medicinal composition, which consists of the following components in percentage by weight: 3.3 to 20 percent of blonanserin, 60 to 85 percent of filler, 5 to 25 percent of disintegrating agent, 2 to 6 percent of adhesive, 0.25 to 5 percent of lubricant and 0.5 to 2 percent of fluidizer. The medicinal composition has the dissolution rate of over 75 percent in a dissolution medium with the pH of 4.0 to 6.0 so as to effectively solve the problem of dissolution rate of blonanserin medicaments.
Owner:泰州万全医药科技有限公司
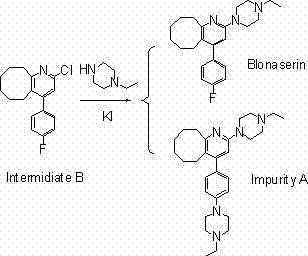
Preparing method of blonanserin intermediate
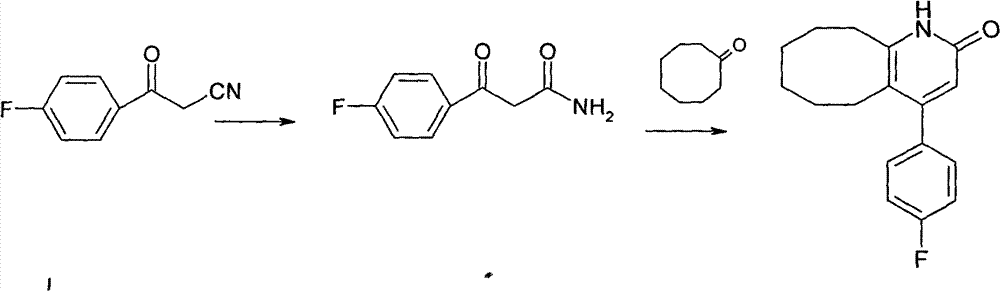
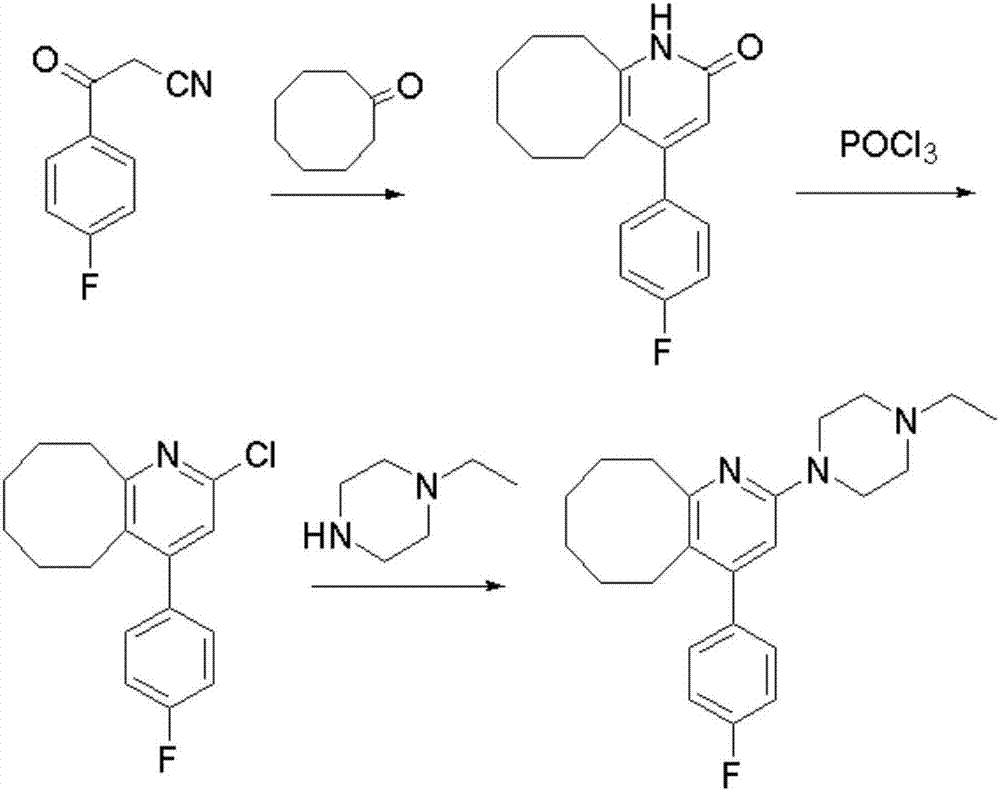
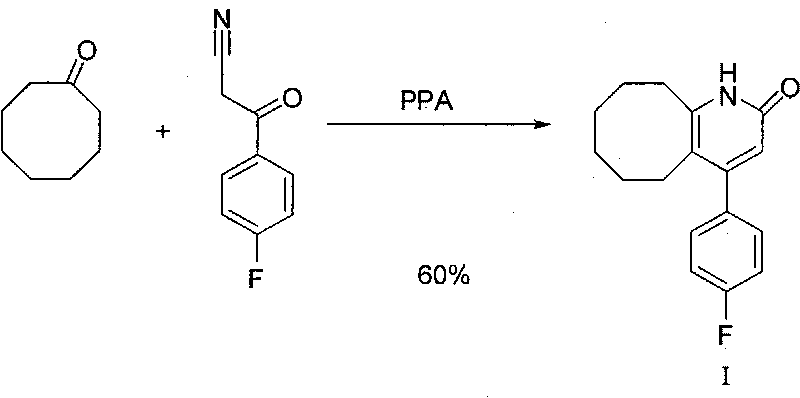
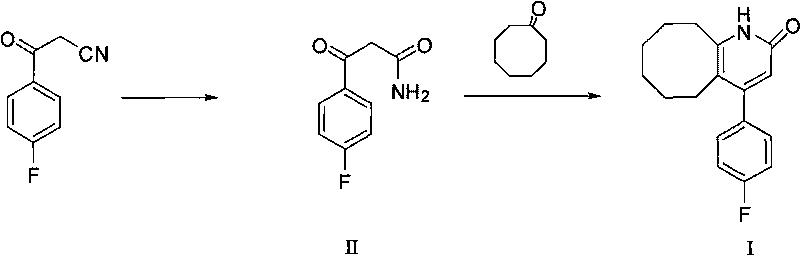
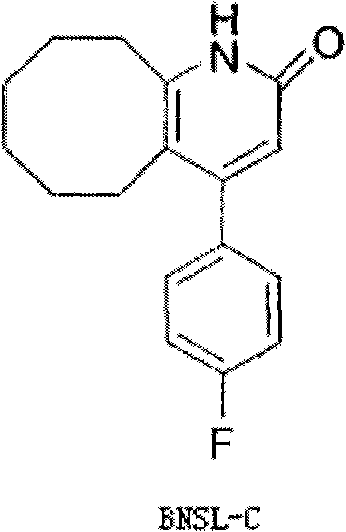
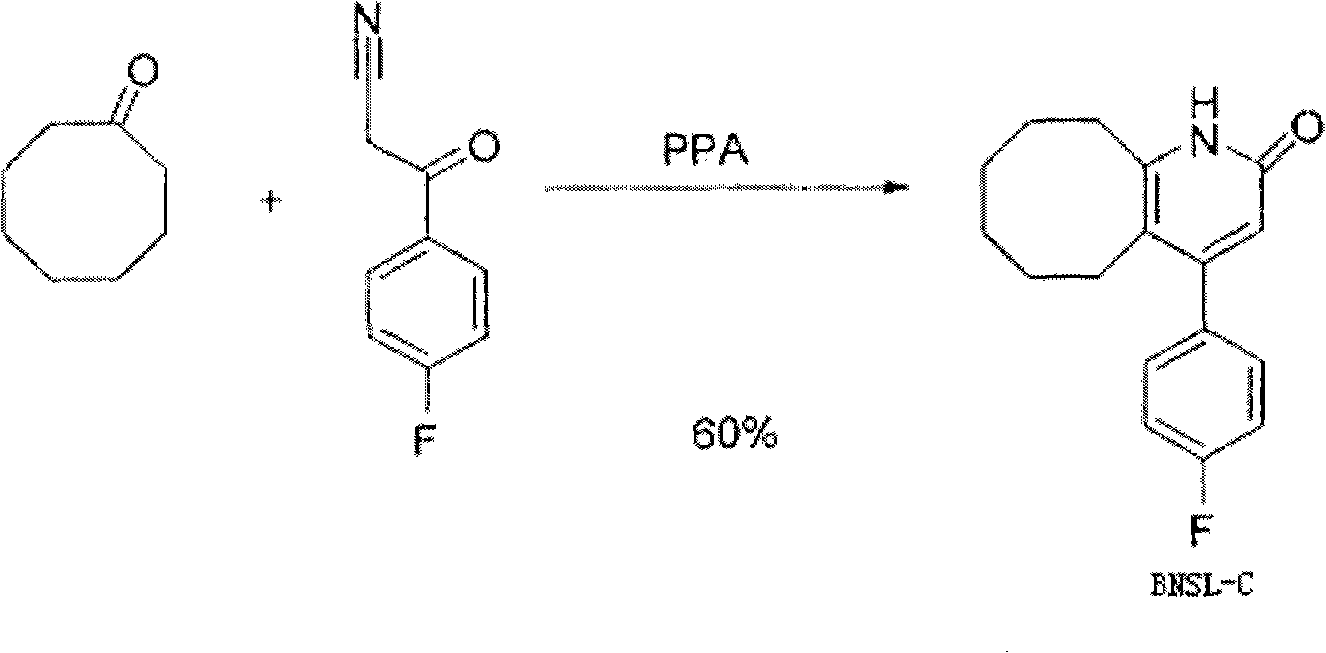
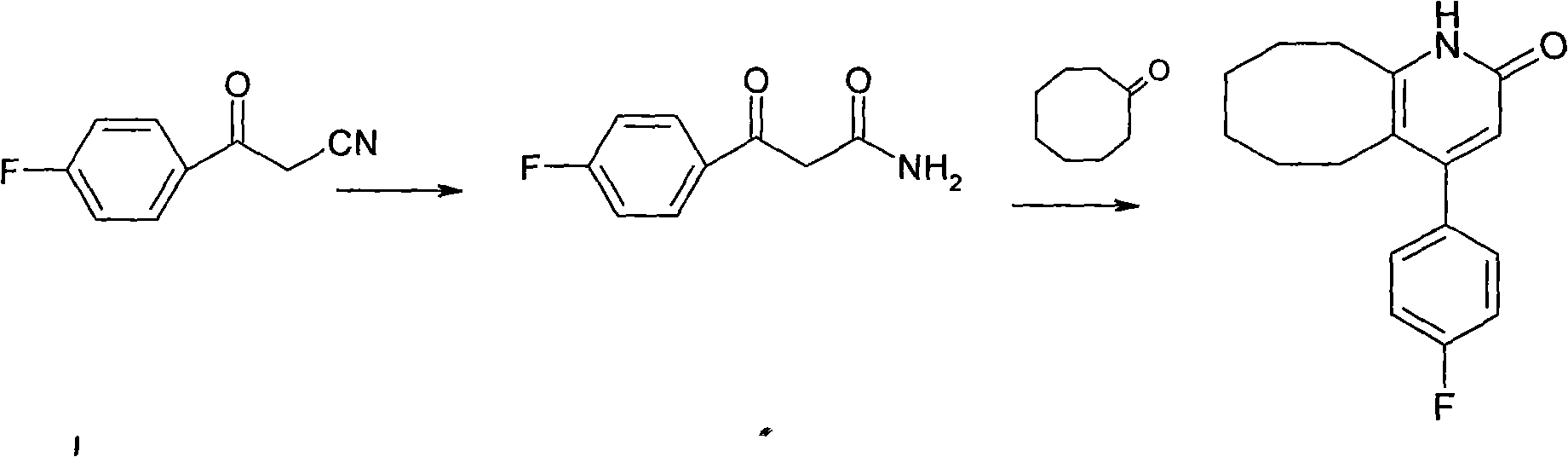
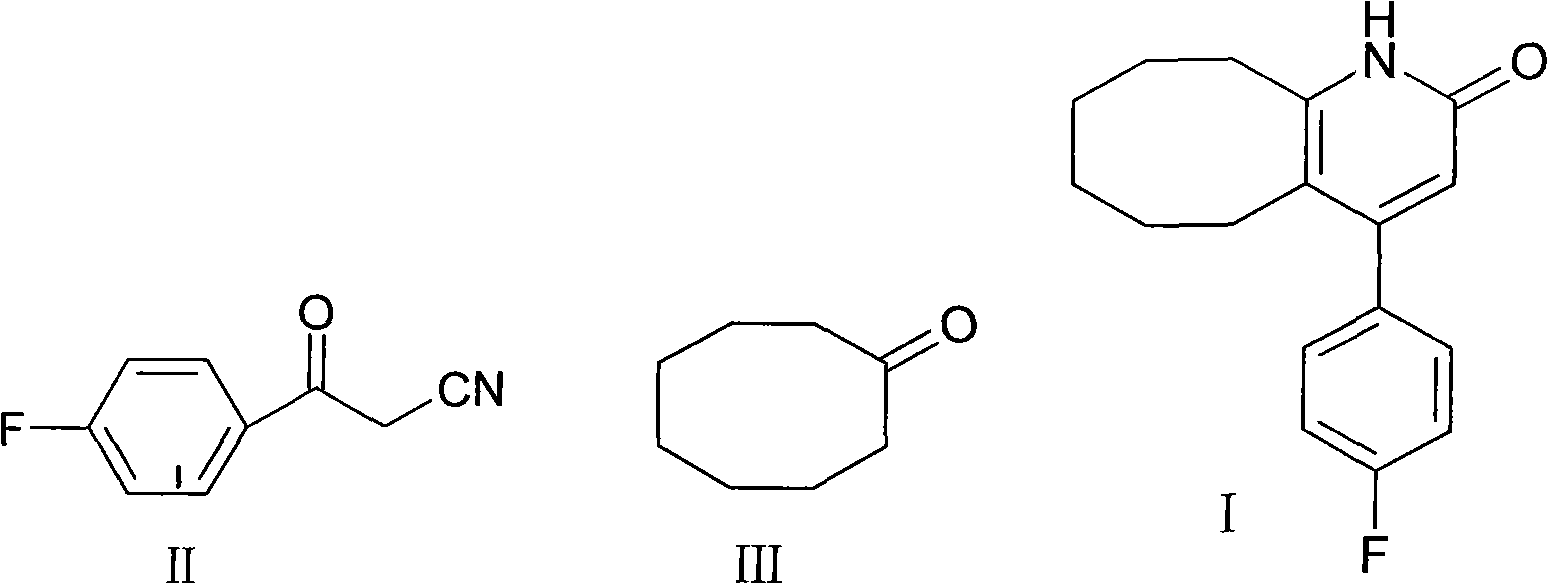
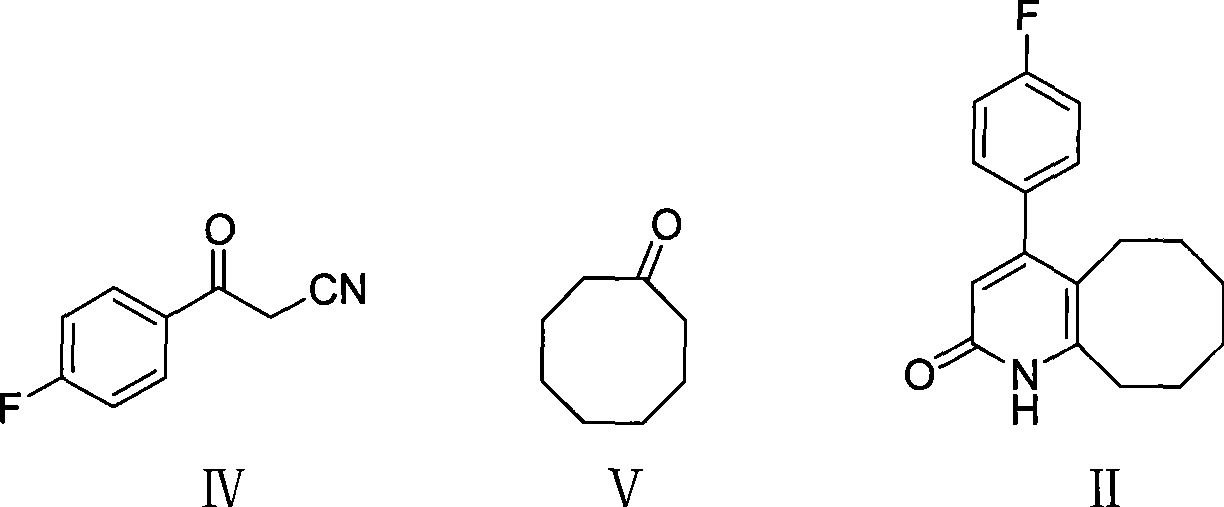
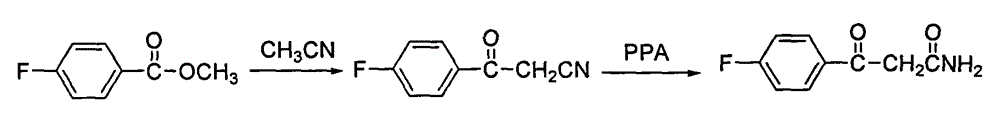
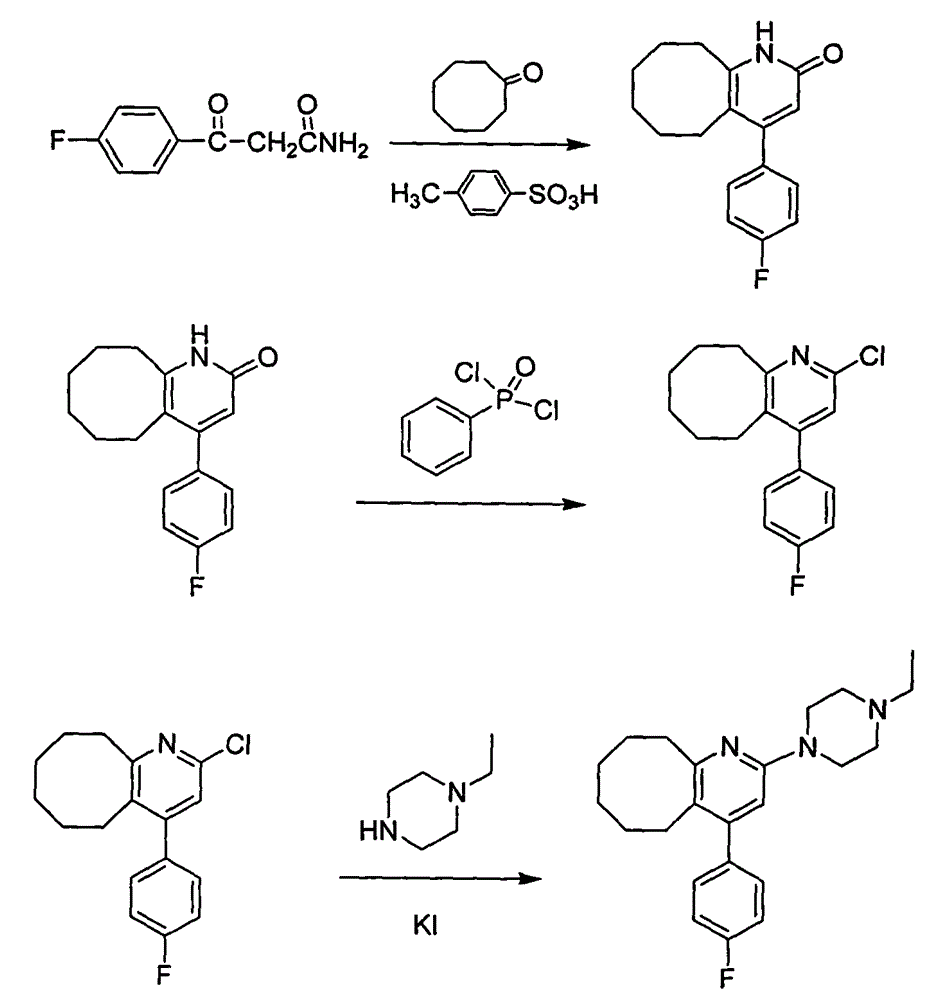
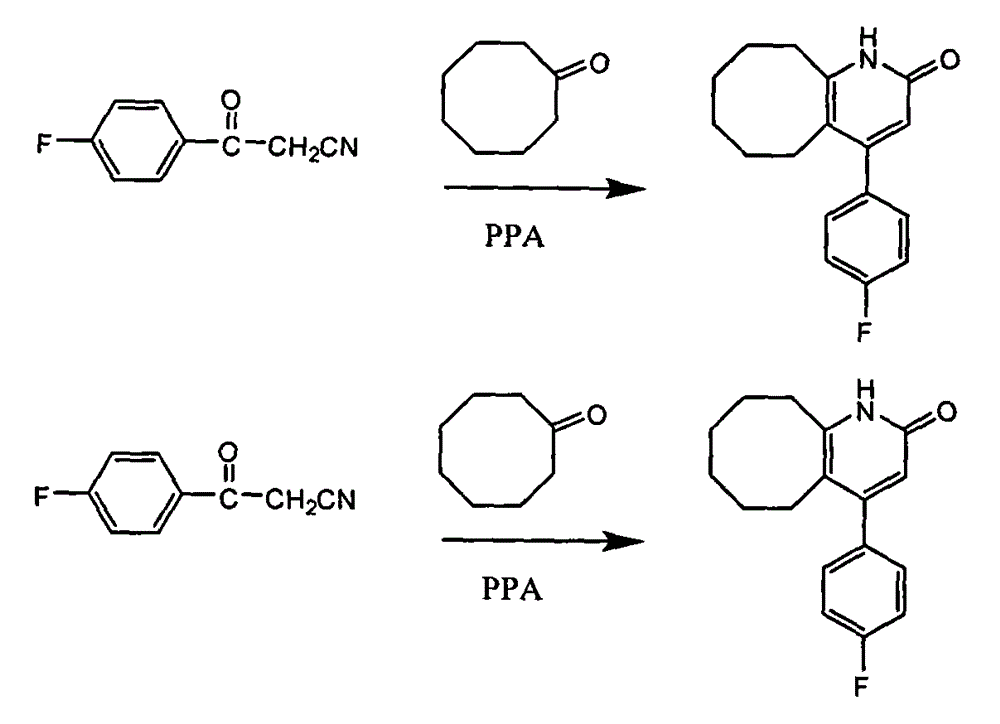
The invention discloses a preparing method of blonanserin intermediate. The method is processed according to the following two steps of: (a) producing 3-(4-fluorophenyl)-3 oxo-propanamide through the reaction of 3-(4-fluorophenyl)-3 oxypropionitrile; and (b) obtaining 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro cyclooctane pyridine-2-(1H)-ketone through the reaction between the 3-(4-fluorophenyl)-3 oxo-propanamide and cyclooctanone. The yield coefficient of the 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro cyclooctane pyridine-2-(1H)-ketone prepared by using the method is increased to about 80 percent from 17 percent.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Blonanserin-contained oral preparation for treating schizophrenia
ActiveCN101766626AImprove solubilityEasy to storeOrganic active ingredientsNervous disorderBlonanserinAdditive ingredient
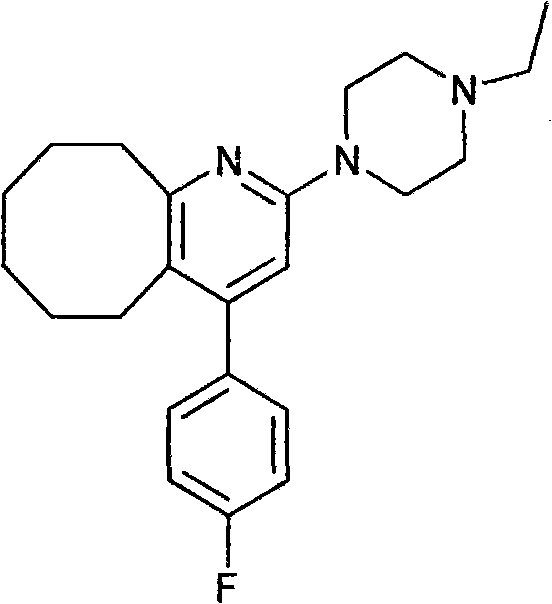
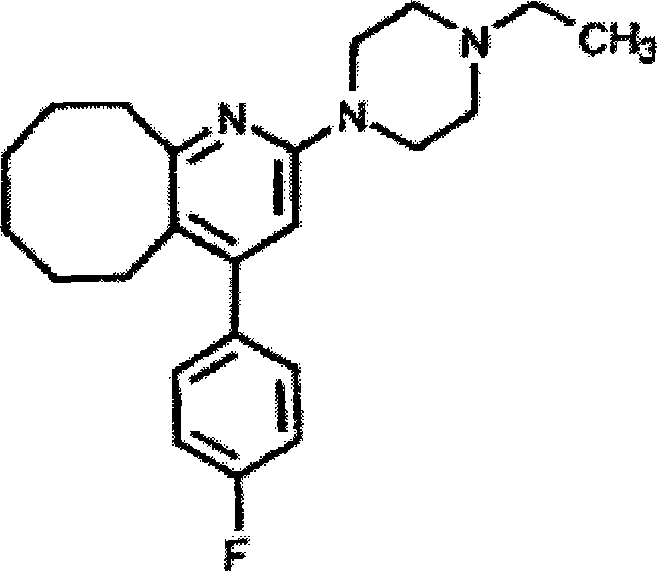
Disclosed is a Blonanserin-contained oral preparation for treating schizophrenia, using a 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine as ingredient, and one or more pharmaceutically acceptable excipients. The preparation method comprises the steps of mixing the 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine and the alternative thinner, adhesive, disintegrant, abhesive and lubricant with an appropriate amount of humectants to prepare soft wood, sieving to prepare wet granules, drying the wet granules, sieving and integrating the granules to prepare oral preparation, wherein, the Blonanserin is 0.1-30 per cent of the preparation by weight. The preparation is applicable to administrate 1-2 times per day in 1-2 preparation units each time, and used for controlling the concentration of the 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine in the plasma of human body, thus enabling the 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine to play a role in treatment.
Owner:LIVZON PHARM GRP INC
Method for preparing Blonanserin intermediate
The invention discloses a method for preparing a Blonanserin intermediate. The method comprises the following two steps in sequence: (a) carrying out catalytic hydrolysis on 3-(4-fluorophenyl)-3-oxopropanenitrile by utilizing sulfuric acids so as to generate 3-(4-fluorophenyl)-3-oxopropionamide; and (b) carrying out catalytic reaction on the 3-(4-fluorophenyl)-3-oxopropionamide and cyclooctanone by utilizing anhydrous p-toluenesulfonic acids so as to generate 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b] pyridine-2(1H)-ketone. According to the method provided by the invention, the yield coefficient of the prepared 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b] pyridine-2 (1H)-ketone is increased from 63.5 percent to 75 percent.
Owner:SHENZHEN WANHE PHARMA
New preparation method of Blonanserin intermediate
ActiveCN102093289AThe reaction steps are simpleSimple post-processingOrganic chemistryBlonanserinPyridine
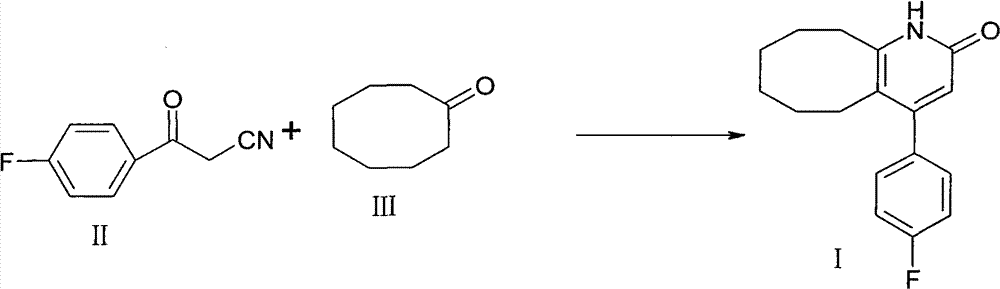
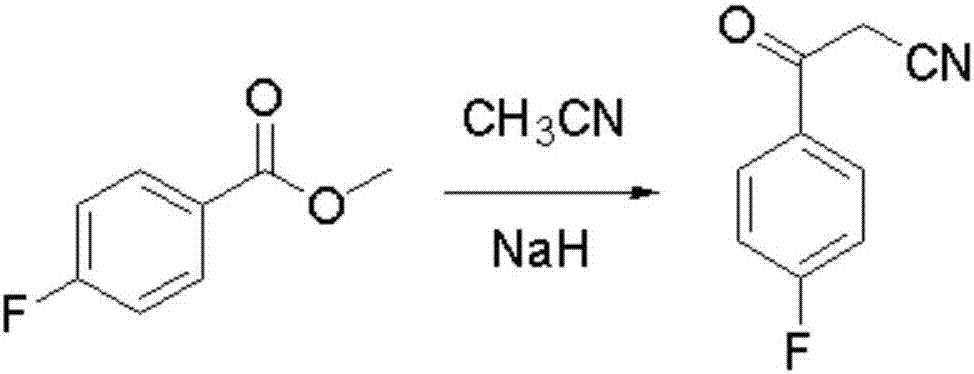
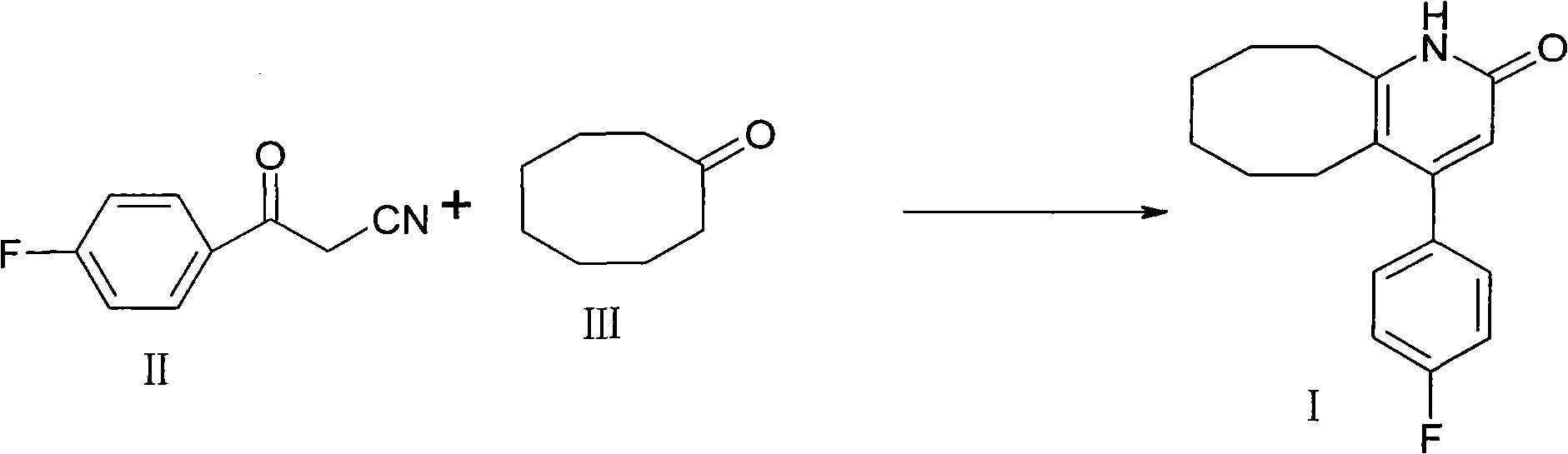
The invention discloses a preparation method of a Blonanserin intermediate. In the method, 4-fluorobenzoylacetonitrile reacts with cyclooctanone to generate 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta pyridine-2(1H)-ketone at a high yield in the presence of a catalyst.
Owner:常州华生制药有限公司
Pharmaceutical composition of blonanserin and preparation method thereof
The invention discloses a [2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5, 6, 7, 8, 9, 10- hexahydro-cycloocta-(b) pyridine), a composition of salts thereof for medical purposes and a preparation method thereof. The pharmaceutical composition has such a stabilizing agent as alkaline substance with a weight not less than 10 percent. The composition has good stability and can block a 5-hydroxytryptamine-2 acceptor and a dopamine-2 acceptor and be applied to the treatment of schizophrenia.
Owner:北京德众万全医药科技有限公司
Oral controlled release pharmaceutical compositions of blonanserin
InactiveUS20130143897A1Substantial bioequivalenceOrganic active ingredientsNervous disorderControl releaseBlonanserin
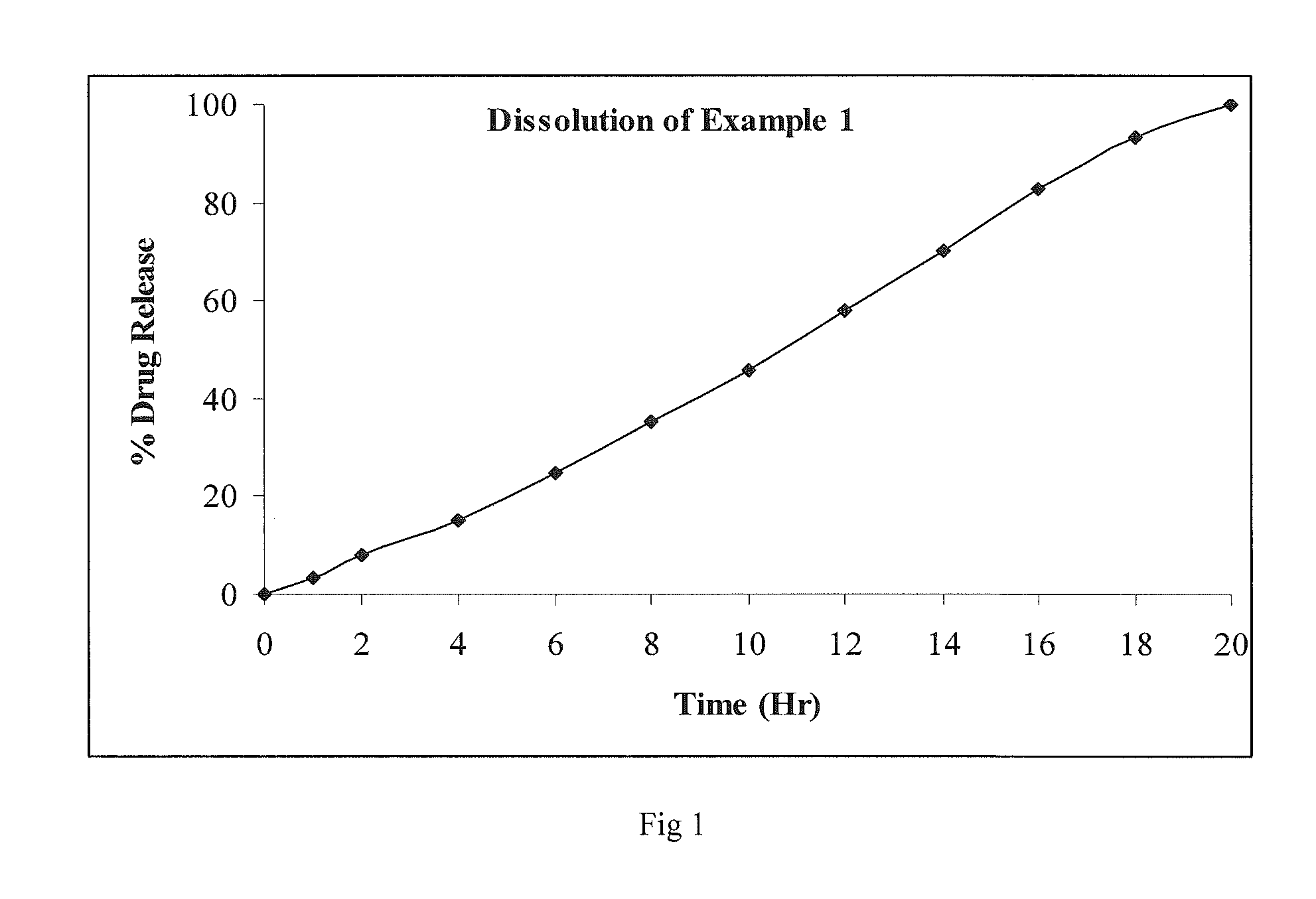
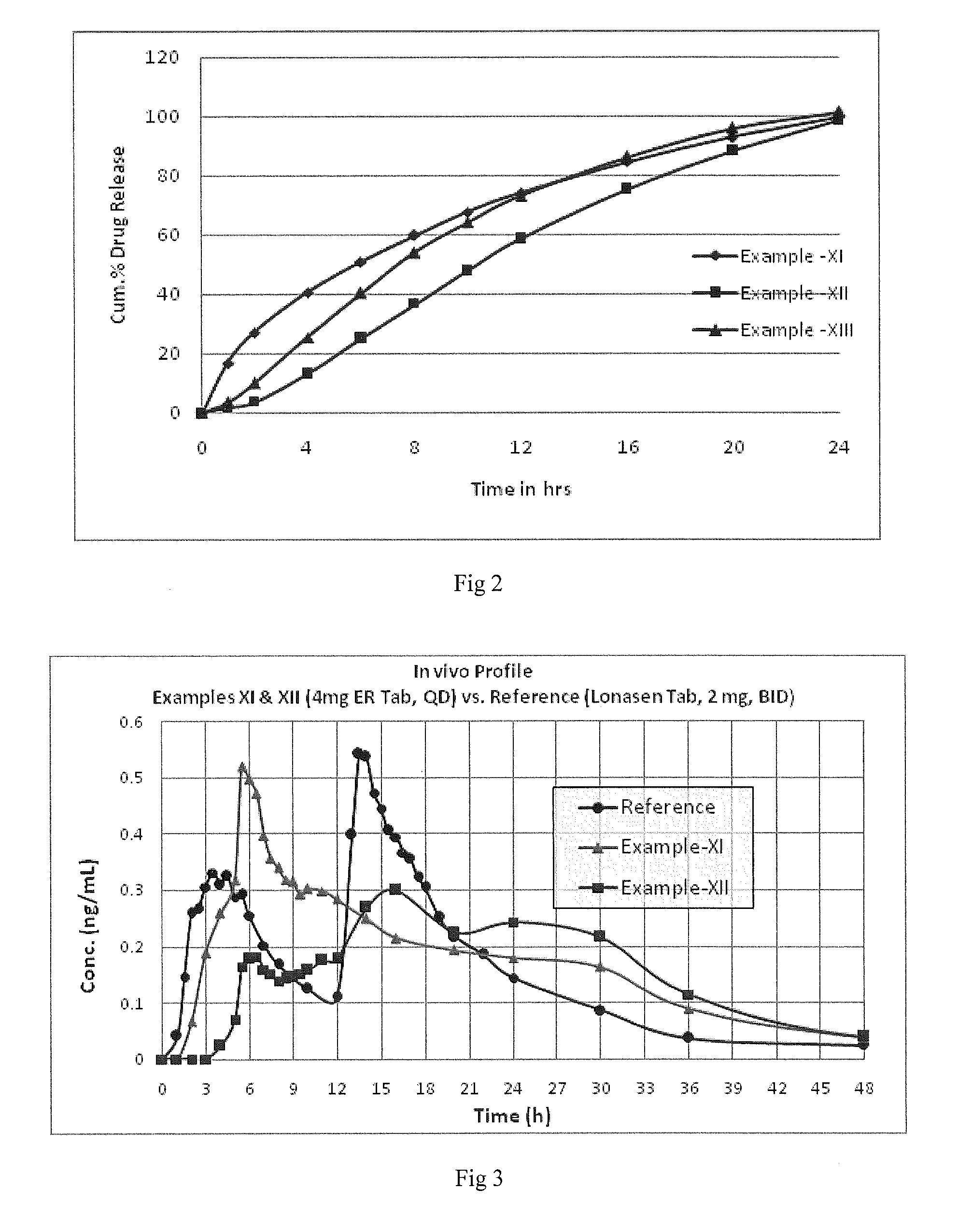
An oral controlled release pharmaceutical composition comprising Blonanserin and release controlling agent(s) and optionally pharmaceutically acceptable excipients is provided. The present invention further relates to a controlled release pharmaceutical composition comprising Blonanserin and release controlling agent(s) such that the composition releases not less than about 80% of Blonanserin within 20 hours, when dissolution is carried out in 900 ml, 0.1 N HCl, USP apparatus Type II (Paddle) at 50 rpm for 20 hrs. The controlled release pharmaceutical composition of the invention releases 50% of Blonanserin between about 4 to 14 hours, when dissolution is carried out in 900 ml, 0.1 N HCl, USP apparatus Type II (Paddle) at 50 rpm.
Owner:LUPIN LTD
Blonanserin tablet and preparation method thereof
ActiveCN102240270AHigh hardnessHigh content of active ingredientsOrganic active ingredientsNervous disorderBlonanserinActive component
Owner:LIVZON PHARM GRP INC
Method for synthesizing Blonanserin
ActiveCN102887856AWide variety of sourcesLow reaction temperatureOrganic chemistrySulfonateSulfonyl chloride
The invention discloses a method for synthesizing Blonanserin, wherein the method comprises the following steps of taking 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridin-2(1H)-one (which is named as an intermediate I) as an initial raw material, enabling the initial raw material to react with a substituted sulfonyl chloride to obtain 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridin-2-substituted sulphonate (which is named as an intermediate II-(1-n)), condensing the intermediate II-(1-n) with N-ethyl piperazine to obtain the Blonanserin, refining the obtained Blonanserin to obtain the Blonanserin with purity of 99.8%. The method provided by the invention prepares the Blonanserin raw material that has high purity and accords with the medicinal use by using a sulfonylation process; the method prepared by the invention has the advantages of high reaction selectivity, high product purity, low solvent toxicity, simple operation and easiness in control; the method provided by the invention is suitable for industrial production.
Owner:HEBEI GUOLONG PHARMA CO LTD
Method for preparing blonanserin
InactiveCN101955459AHigh yieldHigh purityOrganic active ingredientsNervous disorderBlonanserinPharmacodynamic Study
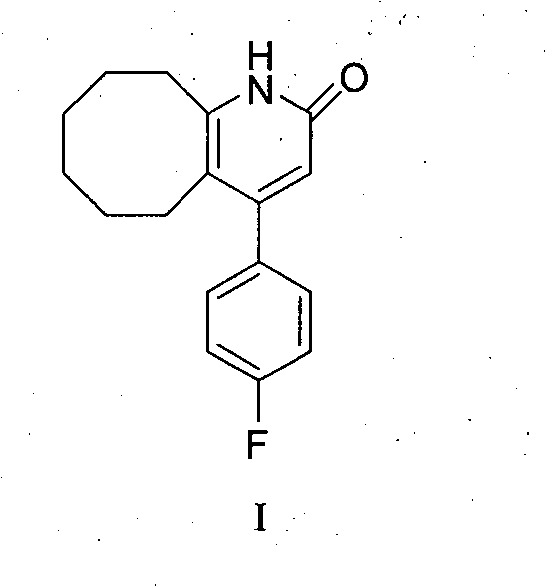
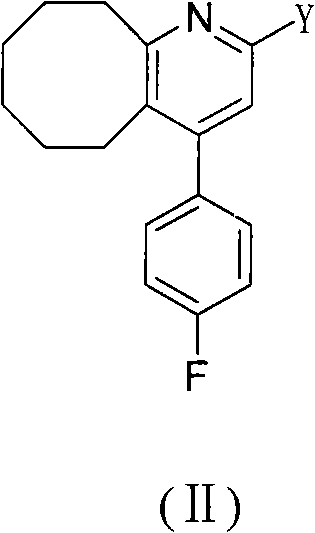
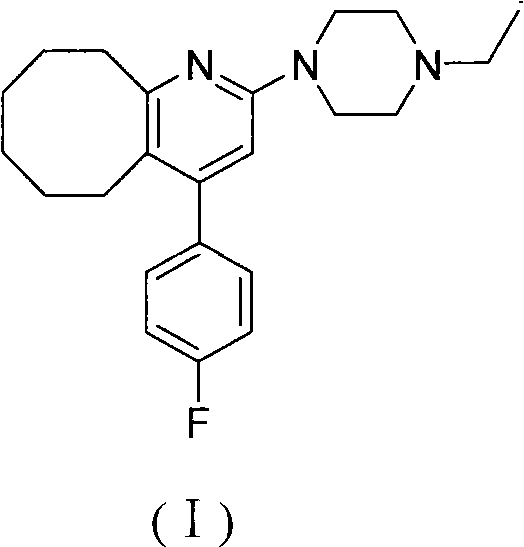
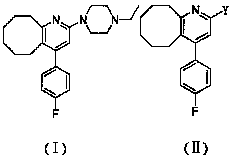
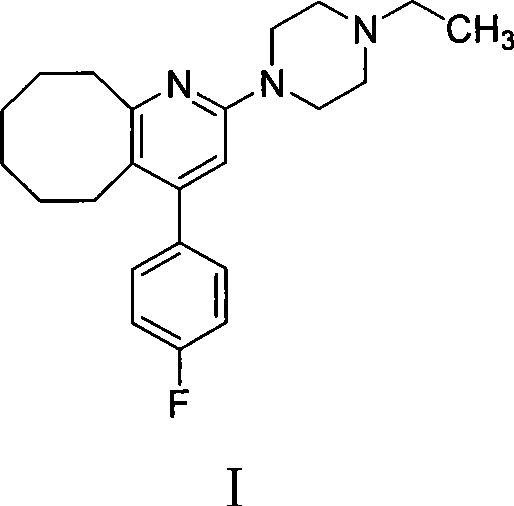
The invention relates to a method for preparing a compound blonanserin in a formula (I), which comprises a step of transforming a compound in a formula (II) and N-ethylpiperazine under the catalysis of base into the compound blonanserin in the formula (I). The blonanserin has extremely valuable pharmacodynamic and treatment properties in the aspect of treating schizophrenia.
Owner:BEIJING D VENTUREPHARM TECH DEV
High-purity blonanserin and preparation method thereof
ActiveCN101531634AThe reaction is easy and controllableEasy to separate and high purityNervous disorderOrganic chemistryBlonanserinBiochemical engineering
The invention discloses a high-purity 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5, 6, 7, 8, 9, 10-hexahydro-cycloocta-(b) pyridine and a preparation method thereof. The target product of the invention can be applied to the treatment of schizophrenia, with higher safety and effectiveness. The preparation method has mild reaction condition and simple post treatment, and can obtain high-purity final products and is easier to implement industrialized production.
Owner:AVENTIS PHARMA HAINAN
Blonanserin pharmaceutical composition and preparation method thereof
InactiveCN105476967AHigh yieldReduce manufacturing costOrganic active ingredientsNervous disorderBlonanserinPharmaceutical drug
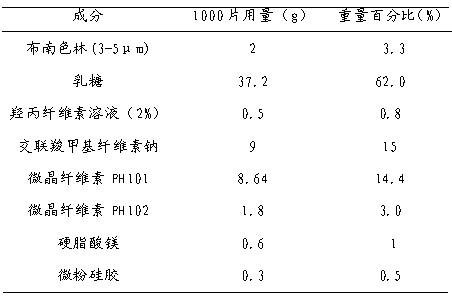
The invention discloses a blonanserin pharmaceutical composition. Mannitol and microcrystalline cellulose are used as filling agents, wherein the weight percentage of the mannitol is 40%-55%, and the weight percentage of the microcrystalline cellulose is 20%-30%. The blonanserin pharmaceutical composition is high in stability and has evident advantages of product yield increase, cost reduction, industrialization realization and clinical application, dissolubility can be effectively improved, and bioavailability is improved evidently.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Method for preparing high-purity blonanserin
The invention relates to a new preparation method for blonanserin shown as a formula (I) in the specification. The provided new method for preparing high-purity blonanserin shown as the formula (I) is economic and effective. The crude product of the formula (I) compound obtained through the method possesses the purity of 99.5%, and blonanserin with the single-impurity content less than 0.1% is obtained after being refined.
Owner:高瑞耀业(北京)科技有限公司 +2
Blonanserin dispersible tablet and its preparation method
InactiveCN102727453ADisintegrates quicklyUniform disintegrationOrganic active ingredientsNervous disorderBlonanserinActive ingredient
The invention discloses a blonanserin-containing dispersible tablet combination and its preparation. The dosage form can be rapidly and uniformly dispersed in water to form a suspension, and absorption of the hard-soluble bulk drug blonanserin can be effectively raised.
Owner:北京德众万全医药科技有限公司
Method for preparing crystal form A of blonanserin
InactiveCN101747274ALow toxicityProduction and preparation process safetyNervous disorderOrganic chemistryAcetic acidBlonanserin
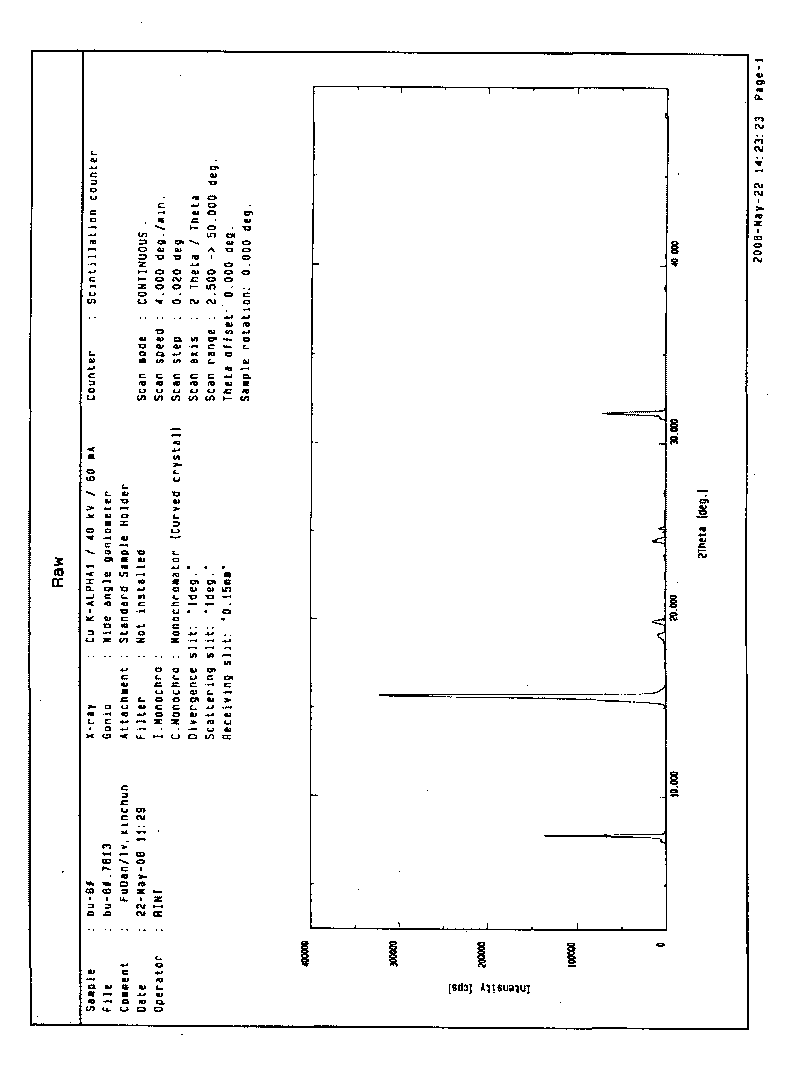
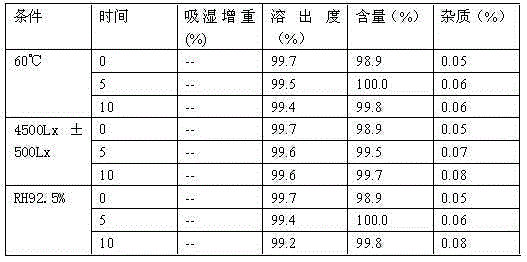
The invention provides a method for preparing crystal form A of medicament 2-ethylpiperazine-4-(4-fluorobenzene)-5,6,7,8,9,10-hexahydro cyclooctane pyridine for treating atypical psychosis. The method is characterized by comprising the following steps of: ensuring that the 2-ethylpiperazine-4-(4-fluorobenzene)-5,6,7,8,9,10-hexahydro cyclooctane pyridine is dissolved in acetone, isopropanol or ethyl acetate by heating, and then obtaining the crystal form A through recrystallization. The total relevant material (PHLC determination) of the crystal form A of the 2-ethylpiperazine-4-(4-fluorobenzene)-5,6,7,8,9,10-hexahydro cyclooctane pyridine prepared through the method can reach lower than 0.3 percent, and single impurity is lower than 0.1 percent.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
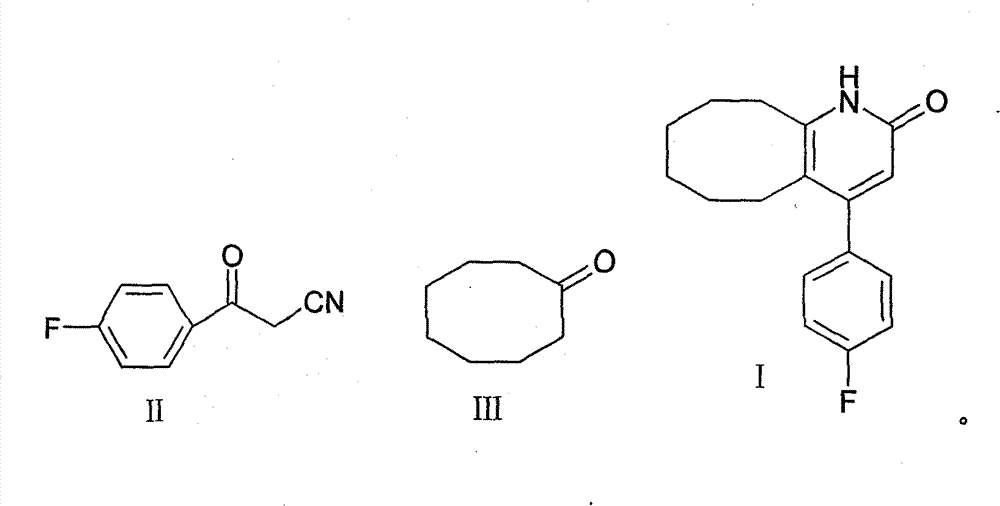
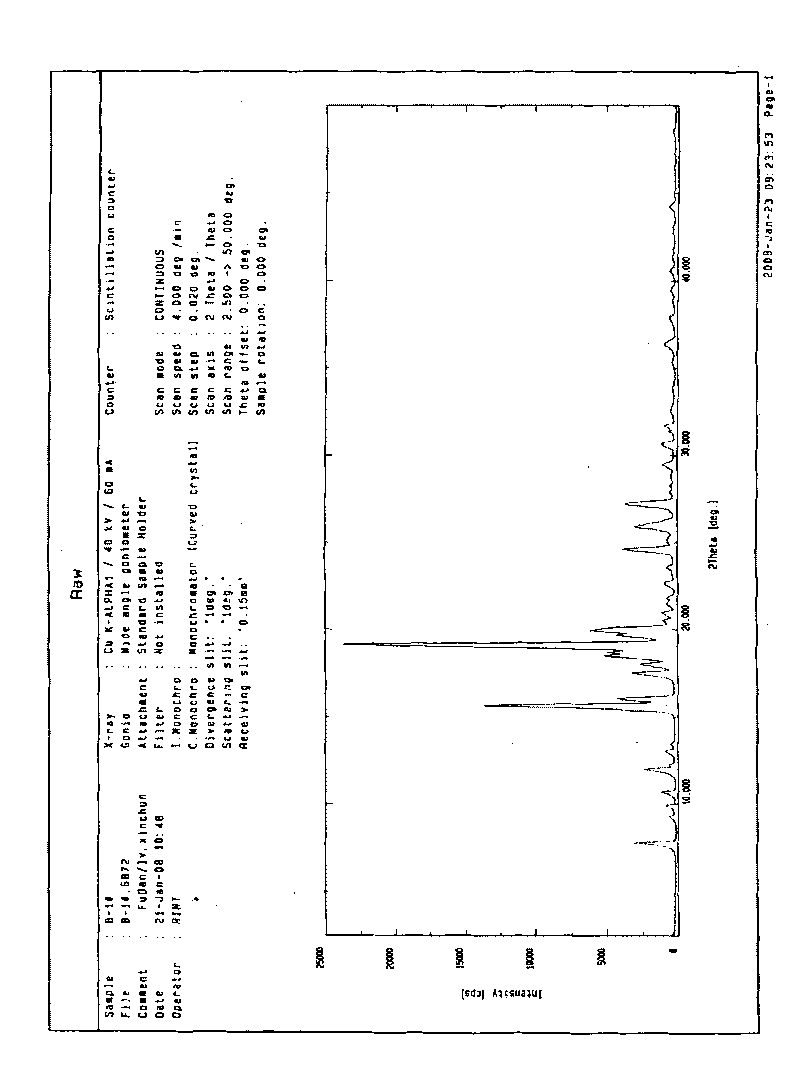
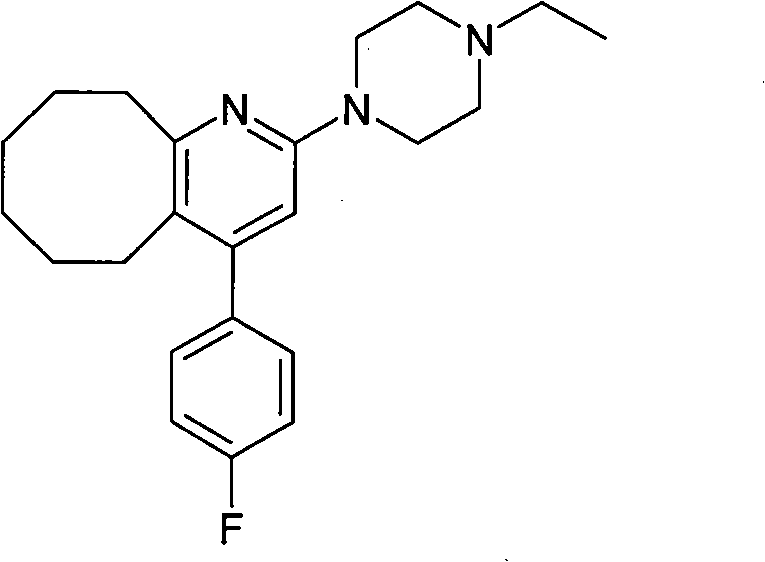
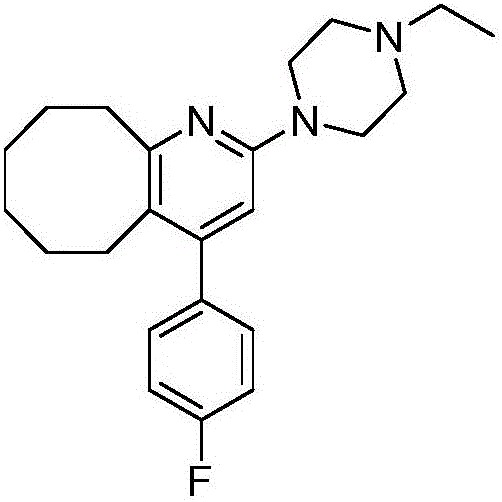
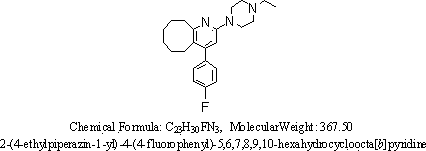
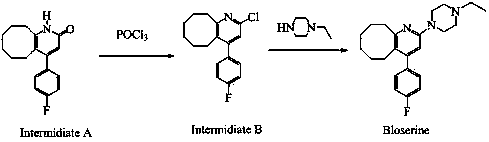
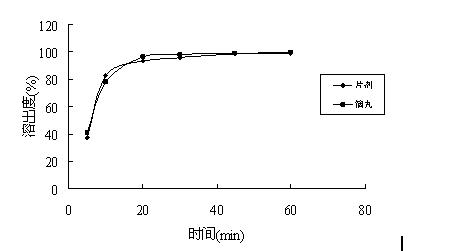
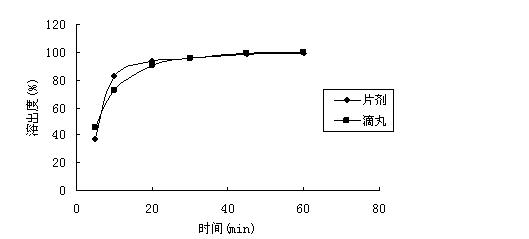
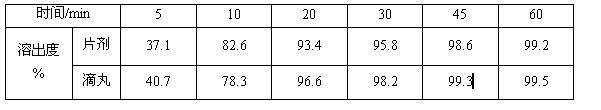
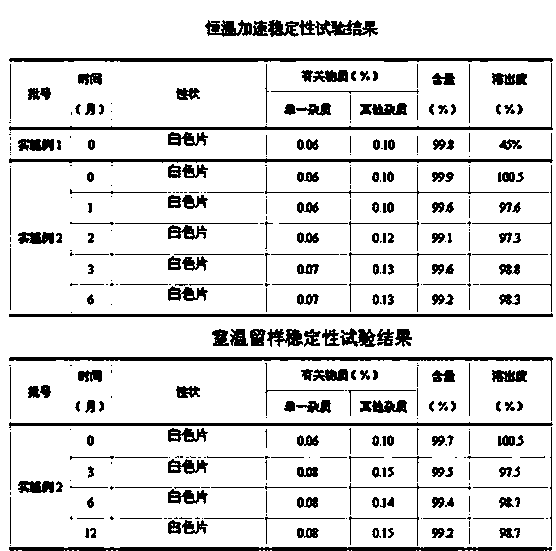
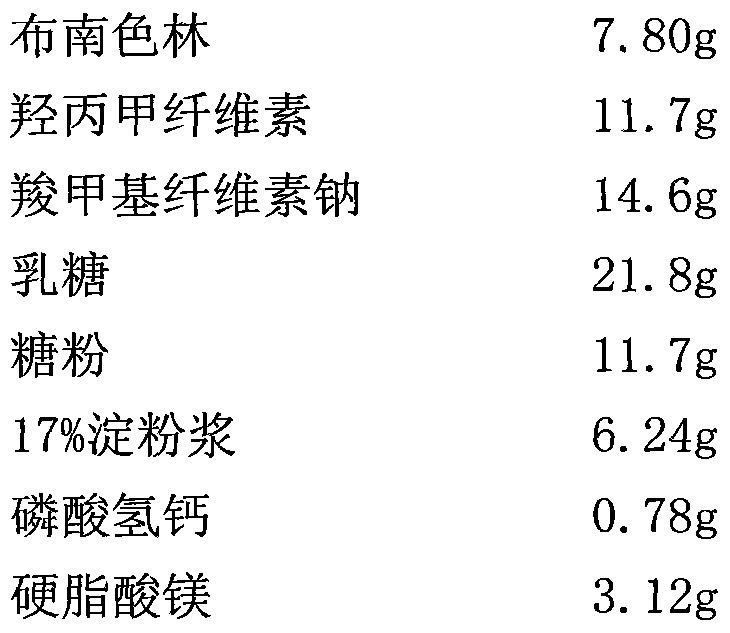
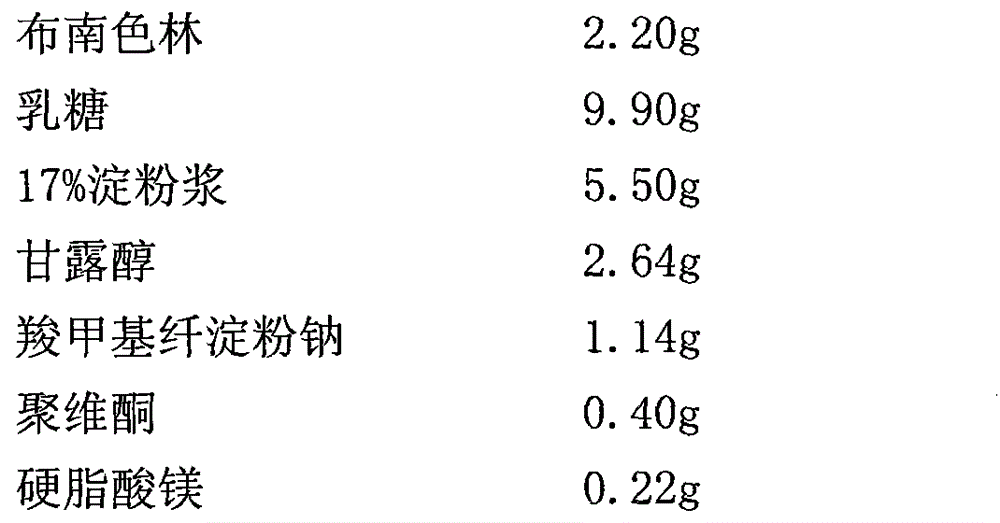
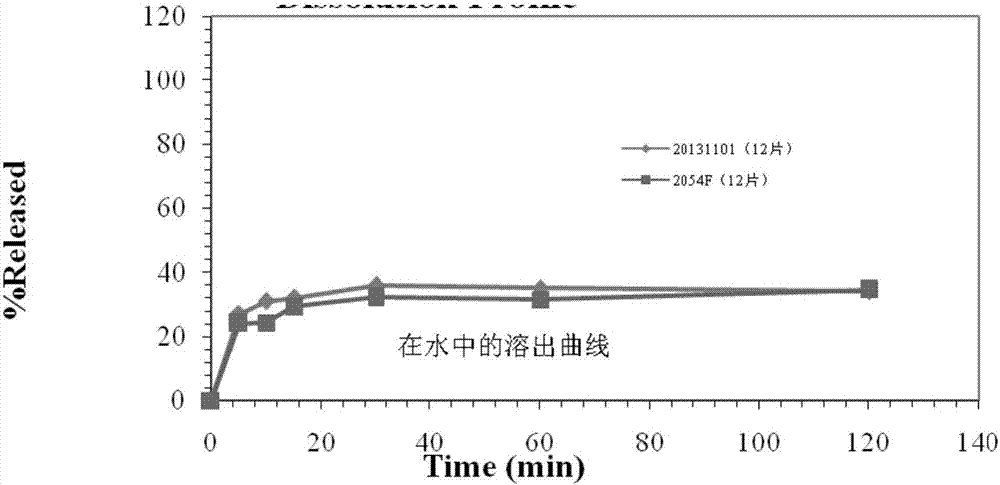
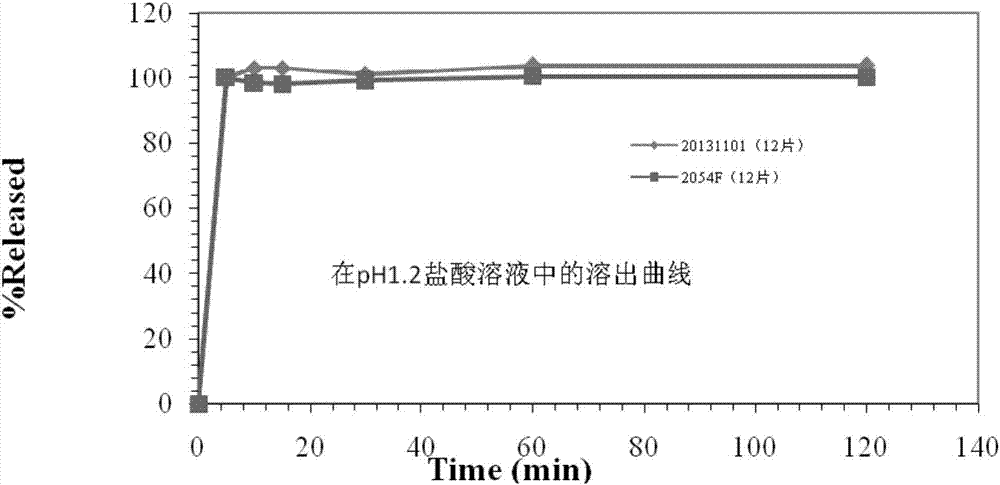
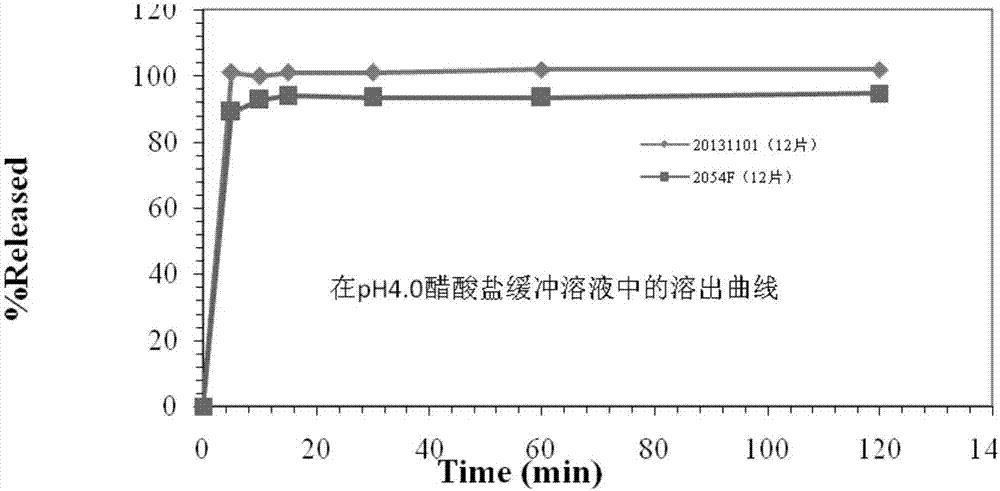
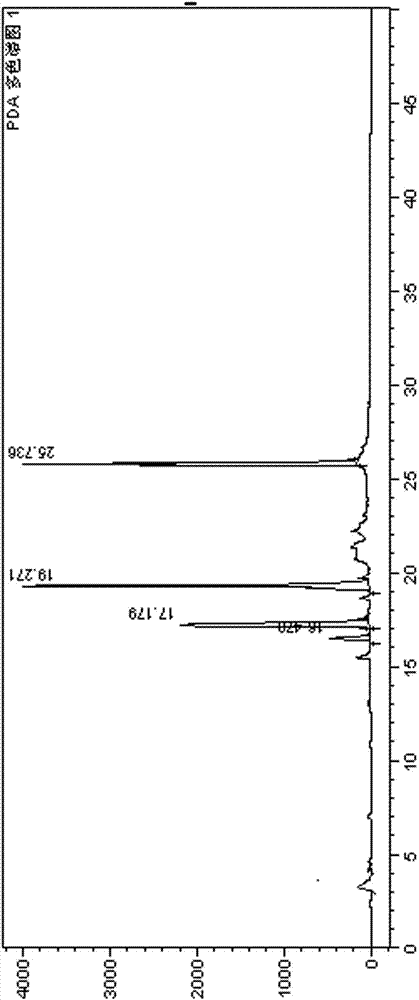
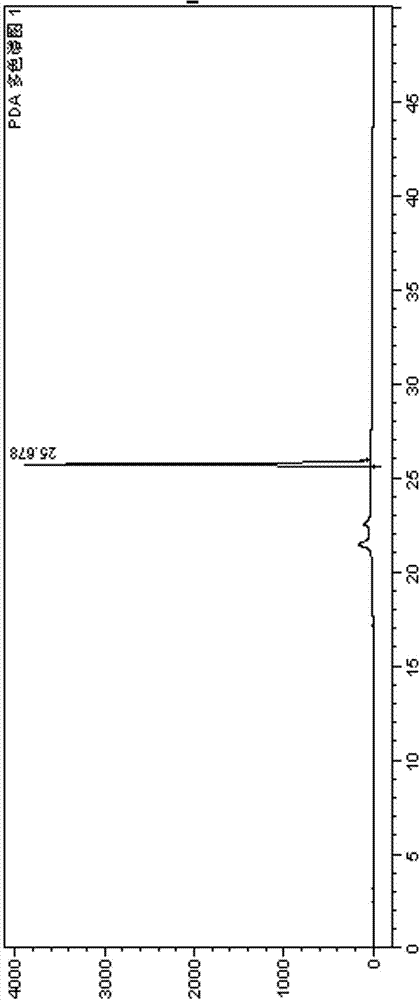
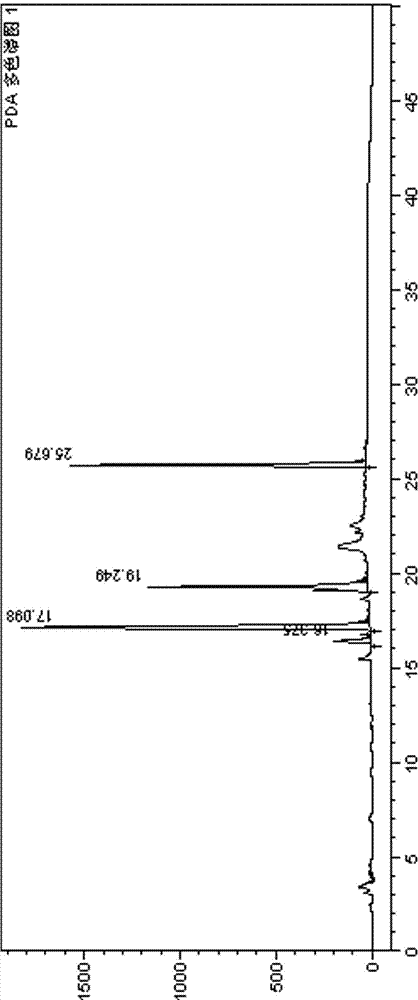
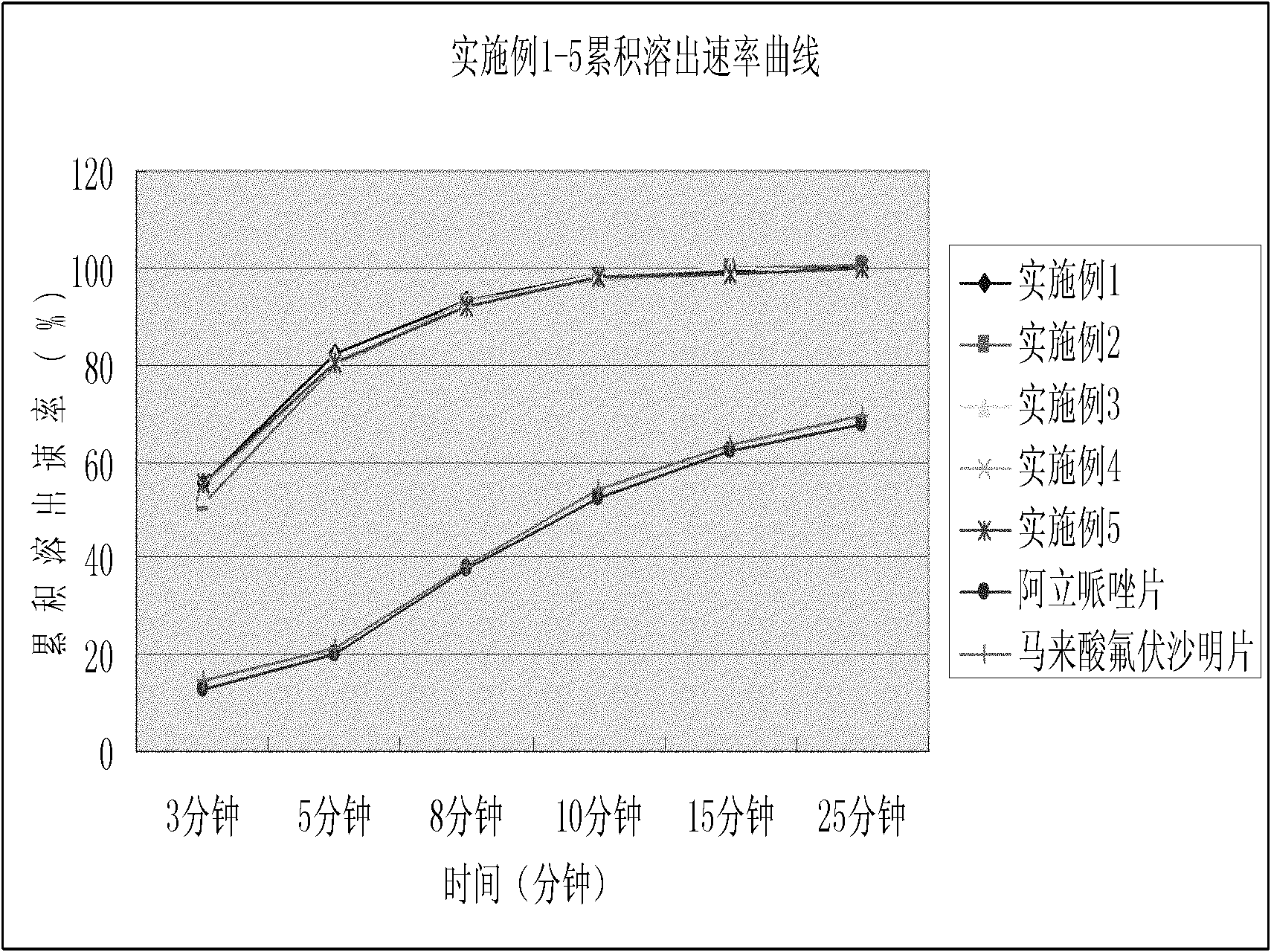
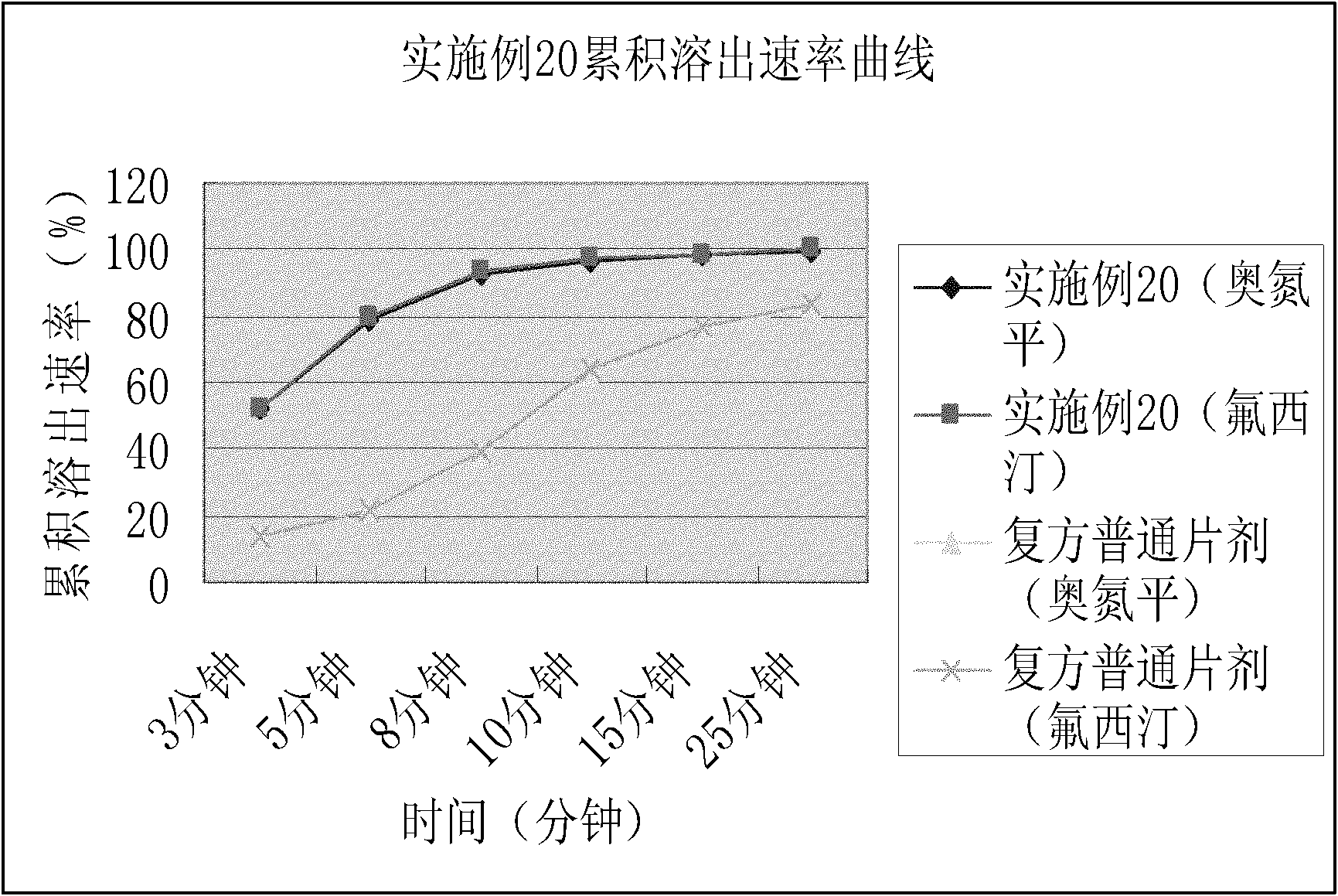
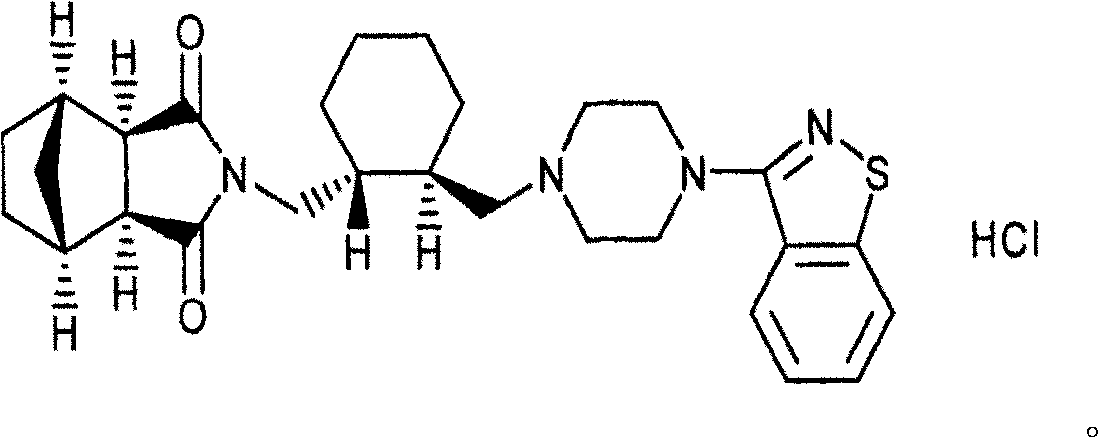
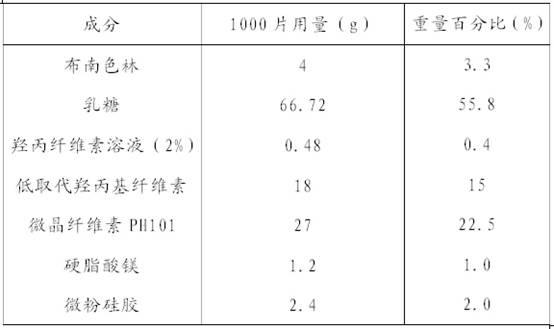
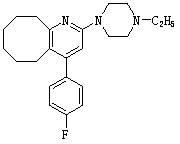
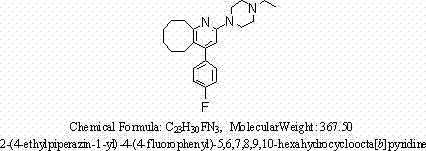
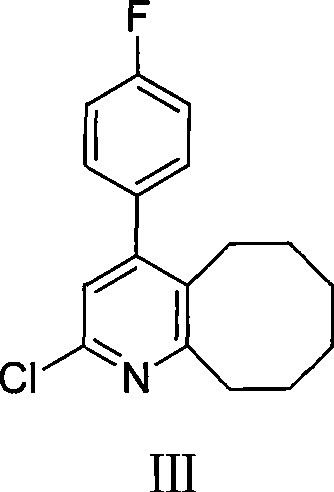
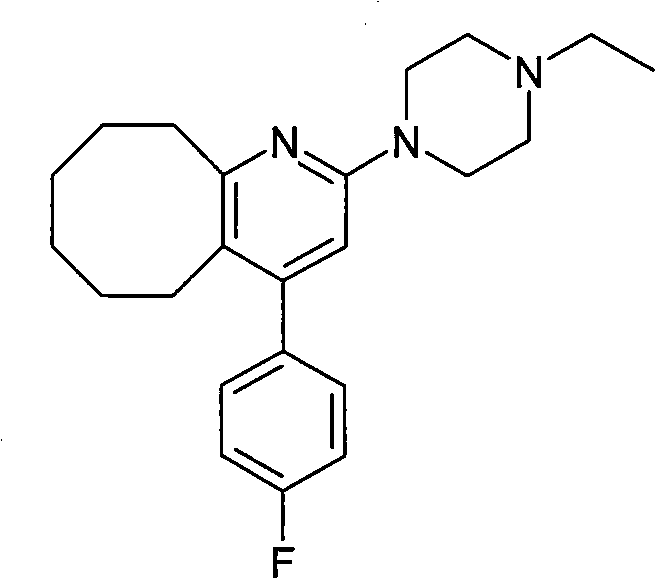
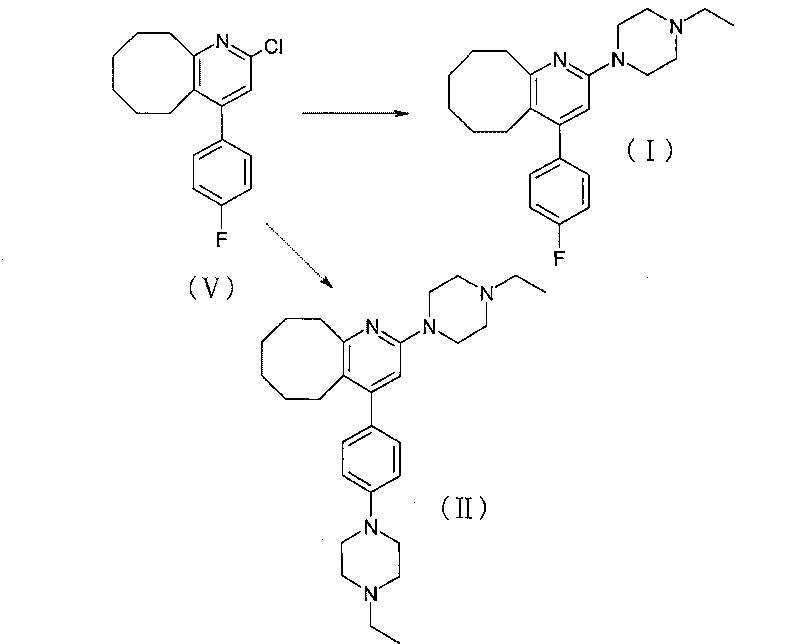
2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine (blonanserin, blonanserin) and composition thereof
ActiveCN101619039AImprove the blocking effectHigh selectivityOrganic active ingredientsNervous disorderBlonanserinPyridine
The invention relates to a crystal of 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine (blonanserin, Blonanserin) and a preparation method thereof. The invention also relates to a blonanserin pharmaceutical composition containing the crystal and an application of the new crystal to the manufacture of medicines for curing schizophrenia.
Owner:LIVZON PHARM GRP INC
New preparation method of Blonanserin intermediate
ActiveCN102093289BThe reaction steps are simpleSimple post-processingOrganic chemistryBlonanserinPyridine
The invention discloses a preparation method of a Blonanserin intermediate. In the method, 4-fluorobenzoylacetonitrile reacts with cyclooctanone to generate 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta pyridine-2(1H)-ketone at a high yield in the presence of a catalyst.
Owner:常州华生制药有限公司
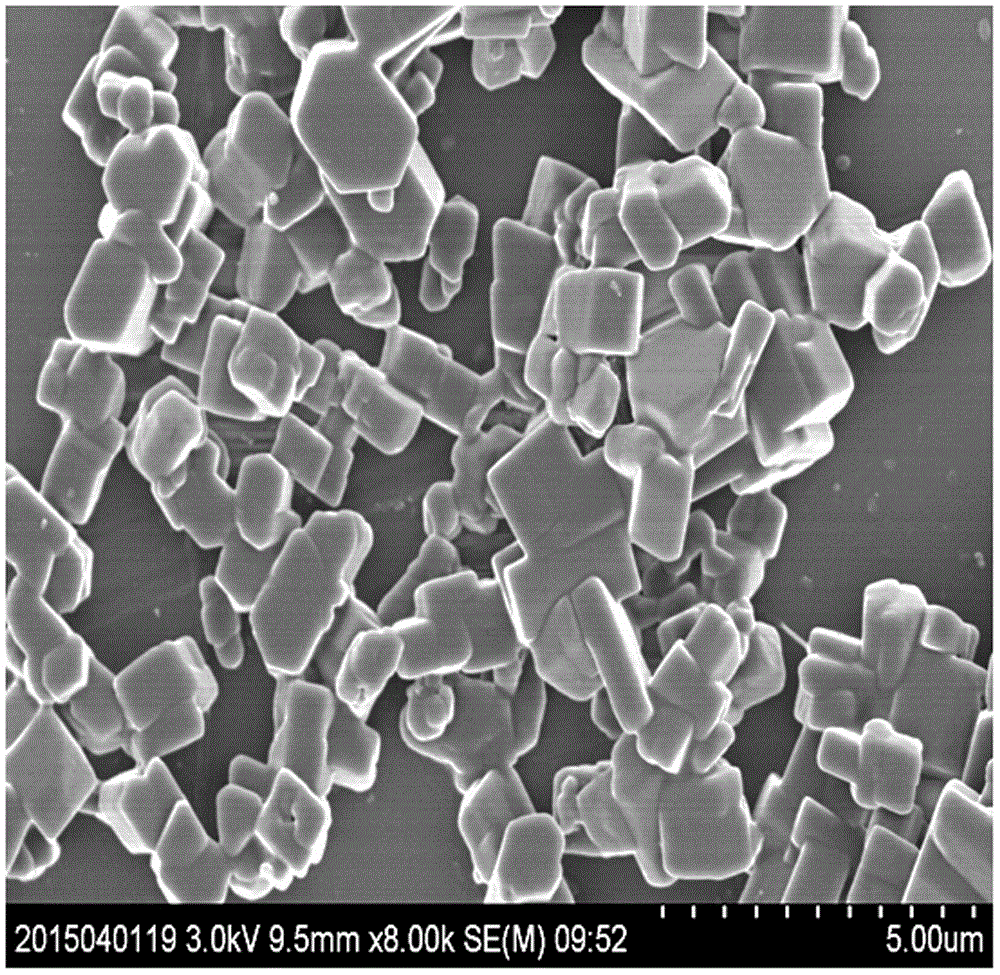
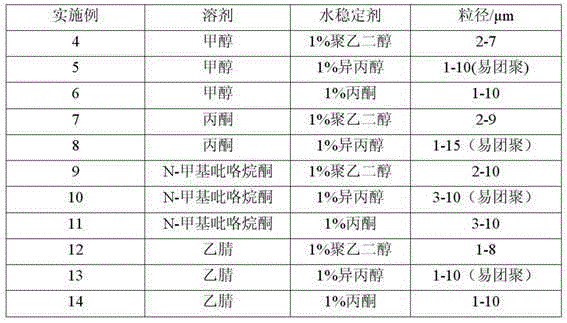
Method for preparing blonanserin micron drug through solvent method
ActiveCN105012240ANo change in crystal formSmall particle sizeOrganic active ingredientsPowder deliveryAdjuvantOrganic solvent
The present invention discloses a method for preparing a blonanserin micron drug through solvent method. According to the method, under an ultrasonic wave condition, a certain amount of blonanserin is dissolved in an organic solvent, and the obtained solution is slowly added to a water stabilizer with a certain concentration in a dropwise manner; the affinity of the organic phase on the water is more than the affinity of the organic phase on the blonanserin, such that the blonanserin is slowly diffused into the water along with the organic phase; and the concentration of the blonanserin in the solution is increased, such that the crystal nucleus is formed and precipitated so as to obtain the blonanserin micron particles with a particle size of 1-10 [mu]m, wherein the particle size is uniform, the specific surface area is increased, and the bioavailability of the blonanserin drug is easily improved. According to the present invention, the operation is simple, other drug adjuvants are not introduced, the equipment investment is low, and the yield is high.
Owner:HEBEI GUOLONG PHARMA CO LTD
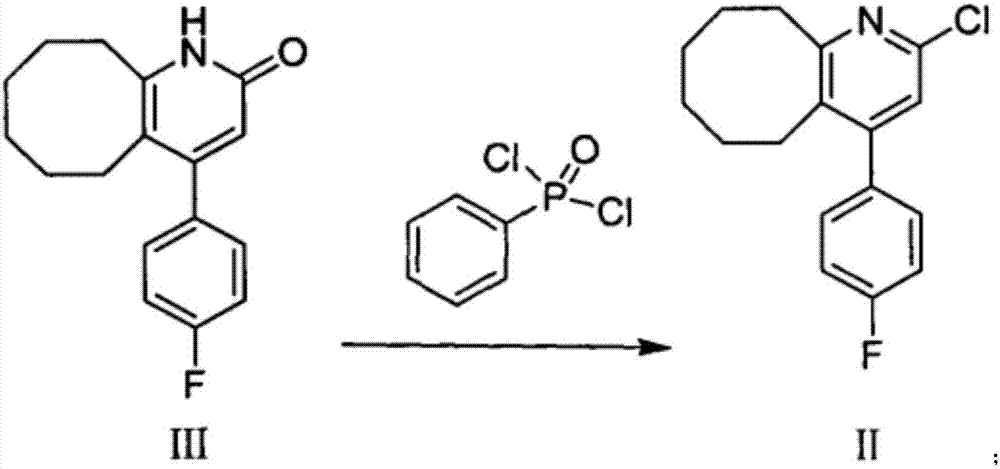
Blonanserin and preparation method thereof
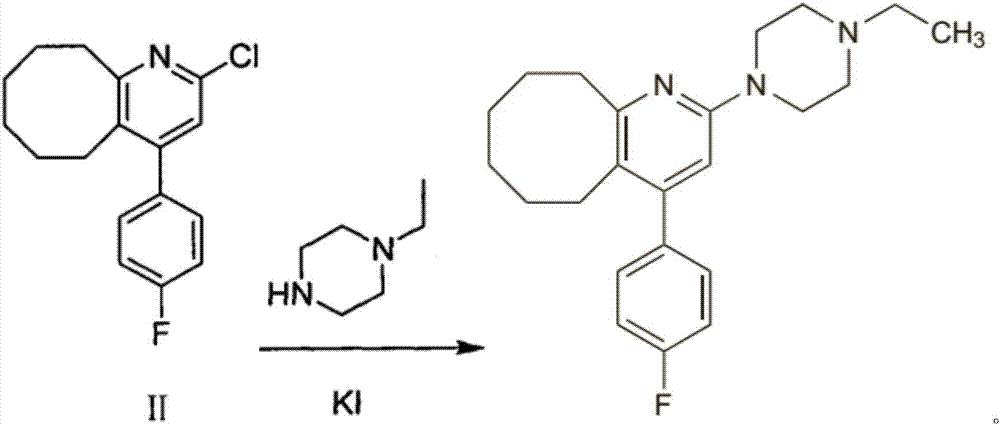
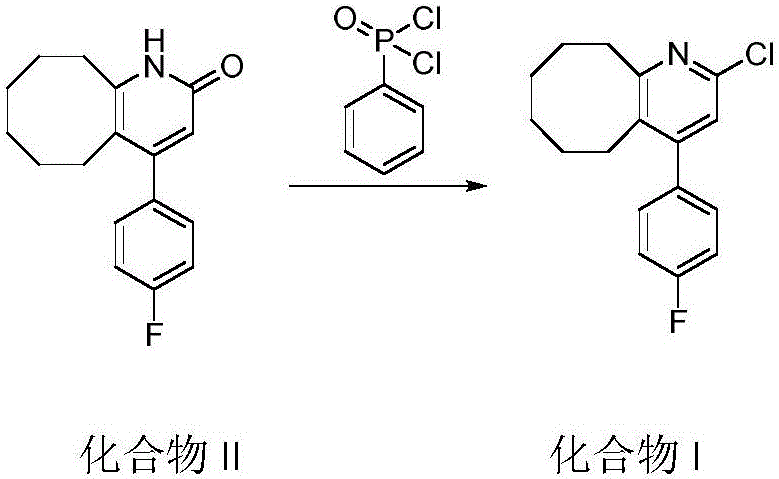
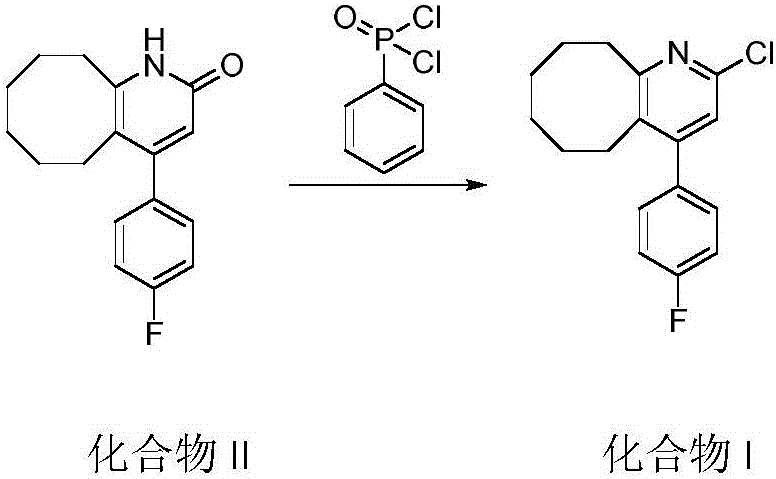
The invention relates to Blonanserin and a preparation method thereof. The preparation method comprises the following specific steps that firstly, the compound shown in the formula III is reacted with a chlorinating agent, namely phosphenylic oxychloride, and the compound shown in the formula II is obtained; secondly, in the presence of potassium iodide, the compound shown in the formula II is reacted with N-ethylpiperazine, and the compound shown in the formula I, namely Blonanserin, is obtained. The preparation method has the advantages shown in the description.
Owner:SHENZHEN WANHE PHARMA
Blonanserin intermediate 4-fluorobenzoylacetonitrile synthesis method
ActiveCN107056653AHigh chemical activityMild in natureCarboxylic acid nitrile preparationOrganic compound preparationBlonanserinPotassium tert-butoxide
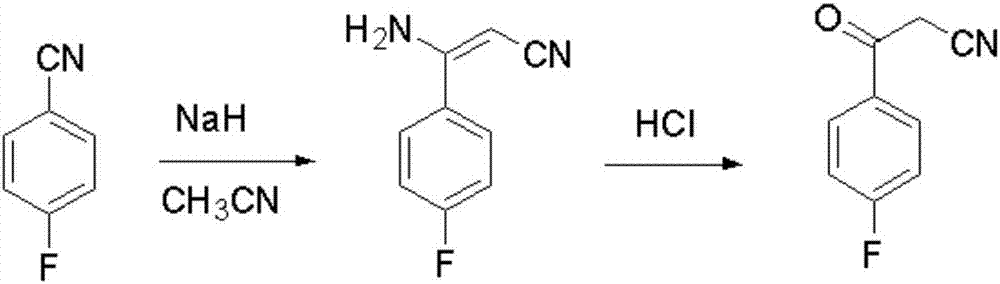
The invention provides a Blonanserin intermediate 4-fluorobenzoylacetonitrile synthesis method. The method comprises the following steps: (1) mixing acetonitrile, t-butanol, p-fluorobenzonitrile and organic solvent A into mixed solution; (2) adding potassium tert-butoxide and the organic solvent A to a reactor, then adding the mixed solution obtained in the step (1), and stirring for reaction to obtain 3-amino-3-p-fluorophenyl acrylonitrile; (3) hydrolyzing the 3-amino-3-p-fluorophenyl acrylonitrile with hydrochloric acid solution to obtain the 4-fluorobenzoylacetonitrile, wherein the organic solvent A is isopropyl ether and / or tetrahydrofuran. According to the method, the potassium tert-butoxide is used as a condensing agent, which greatly improves the safety of the process. The potassium t-butoxide is mild in nature, has hygroscopicity but is nonflammable, and can be used in wider temperature and humidity ranges, and is more conducive to industrial production. And meanwhile, the method is stable in yield and high in obtained product purity.
Owner:LIVZON PHARM GRP INC
Crystal form B of blonanserin and preparing method thereof
ActiveCN101747272AImprove stabilityNot prone to crystallizationOrganic active ingredientsNervous disorderBlonanserinPyridine
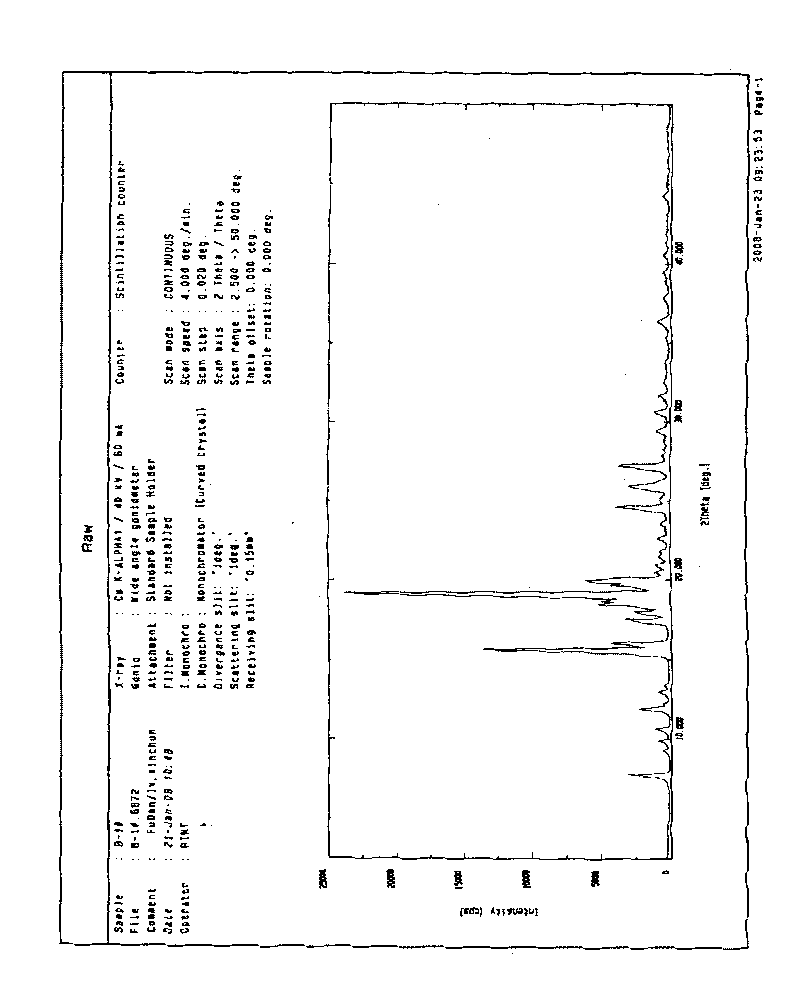
The invention provides a crystal form B of medicament 2-ethylpiperazine-4-(4-fluorobenzene)-5,6,7,8,9,10-hexahydro cyclooctane pyridine, which is characterized in that powder diffraction occurs in the positions of approximately 7.74+ / -0.2, 15.6+ / -0.2, 19.1+ / -0.2 and 31.64+ / -0.2 of a reflecting angle 2 theta through X-ray. The invention also provides a preparing method of the crystal form B, which is characterized by comprising the following steps of: ensuring that the medicament 2-ethylpiperazine-4-(4-fluorobenzene)-5,6,7,8,9,10-hexahydro cyclooctane pyridine is dissolved in ethanol by heating, and then obtaining the crystal form B through recrystallization.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Preparation method of blonanserin intermediate
InactiveCN105837504AReduce lossEliminate the extraction processOrganic chemistryBlonanserinFiltration
Owner:HEBEI GUOLONG PHARMA CO LTD
Method for synthesizing Blonanserin
ActiveCN102887856BWide variety of sourcesLow reaction temperatureOrganic chemistrySulfonateSulfonyl chloride
The invention discloses a method for synthesizing Blonanserin, wherein the method comprises the following steps of taking 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridin-2(1H)-one (which is named as an intermediate I) as an initial raw material, enabling the initial raw material to react with a substituted sulfonyl chloride to obtain 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridin-2-substituted sulphonate (which is named as an intermediate II-(1-n)), condensing the intermediate II-(1-n) with N-ethyl piperazine to obtain the Blonanserin, refining the obtained Blonanserin to obtain the Blonanserin with purity of 99.8%. The method provided by the invention prepares the Blonanserin raw material that has high purity and accords with the medicinal use by using a sulfonylation process; the method prepared by the invention has the advantages of high reaction selectivity, high product purity, low solvent toxicity, simple operation and easiness in control; the method provided by the invention is suitable for industrial production.
Owner:HEBEI GUOLONG PHARMA CO LTD
Blonanserin dropping pill and preparation method thereof
ActiveCN102204893AFit closelySimple prescriptionOrganic active ingredientsNervous disorderPolyethylene glycolDissolution
The invention discloses a blonanserin dropping pill which comprises a mixture of blonanserin and a water-soluble substrate with a weight ratio of 1:2-1:50, wherein each dropping pill contains 0.1 mg-20 mg of blonanserin, and the weight of the dropping pill is 5 mg-150 mg; the water-soluble substrate is polyethylene glycol, poloxamer, polyoxyethylene monostearate (S-40), polyoxyethylene dehydratedsorbitol fatty acid ester, or a mixture of two or more than two compounds. The dropping pill related to by the invention solves the problem of dissolution rate of a hard-soluble drug, and has an in-vitro dissolution rate which fits well with that of commercial preparations in Japan; the dropping pill is easy to swallow, is convenient for dose fractionation, is reasonable for clinical application,and improves the compliance of patients; meanwhile, the preparation method has simple process and no dust, facilitates labor protection, has low production cost, and has good industrial practicality.
Owner:天津市医药集团技术发展有限公司
Blonanserin pharmaceutical composition with improved oral absorptivity
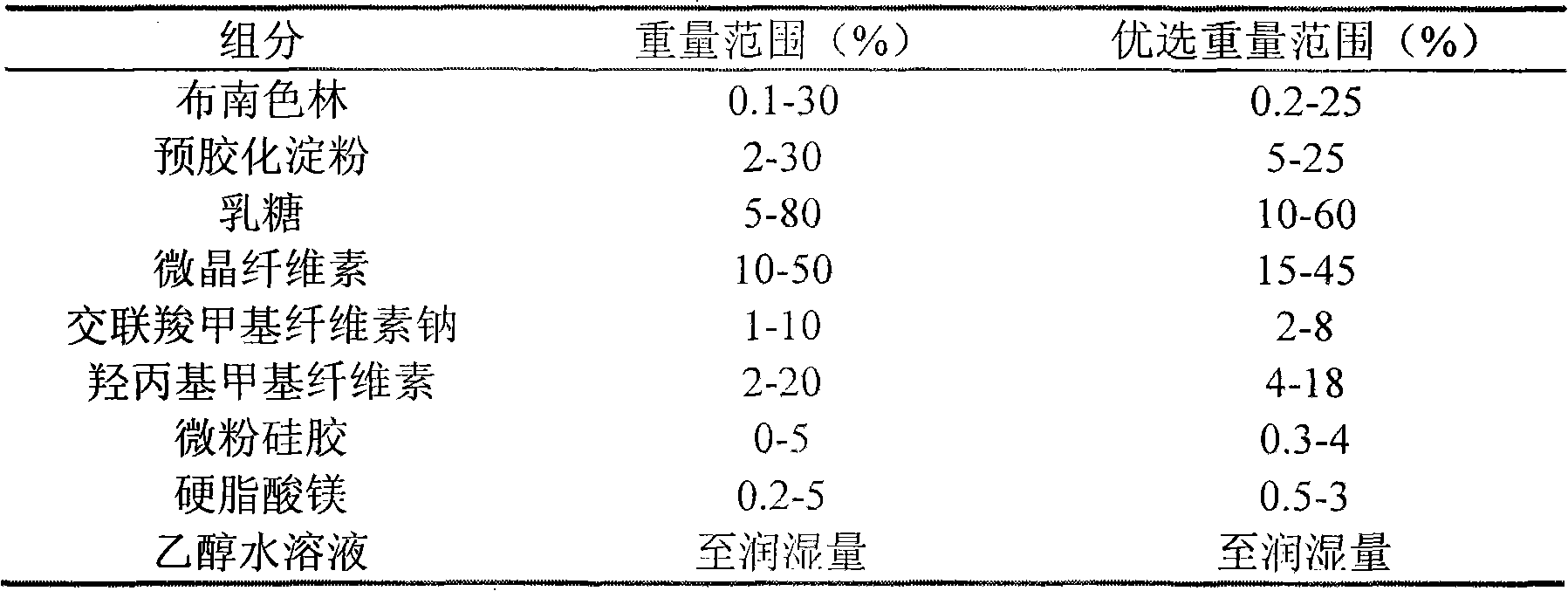
InactiveCN103565762ASubstance increaseDissolution qualifiedOrganic active ingredientsNervous disorderBlonanserinAlcohol
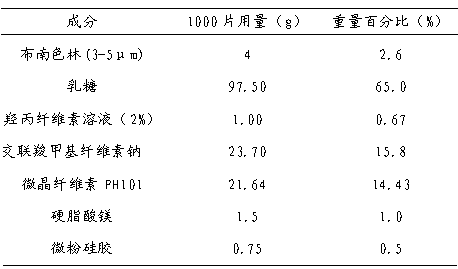
A bonanserin pharmaceutical composition with improved oral absorptivity is characterized by comprising the components by weight: a first part: 4 g of bonanserin and 25 g of alpha cyclodextrin; and a second part: 20 g of cospovidone, 50 g of microcrystalline cellulose, 60 g of lactose, 20 g of pregelatinized starch, 1 g of powdered steatile, 1 g of magnesium stearate and proper amount of a povidone anhydrous-alcohol solution with a ratio of 5%.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Sustained release preparation of blonanserin and preparation method thereof
ActiveCN104306346AReduce the frequency of takingEasy to takeOrganic active ingredientsPharmaceutical delivery mechanismBlood concentrationBlonanserin
The invention relates to a sustained release preparation of blonanserin and a preparation method of the sustained release preparation of the blonanserin. A blonanserin tablet is prepared by mixing slow release particles prepared by slow release components and common components according to the prescription proportion; the mass ratio of the slow release components to the common components ranges from 75:25 to 80:20; the slow release components of the blonanserin tablet mainly comprise, by mass, 8.00%-12.0% of the blonanserin, 15.0%-20.0% of hydroxypropyl methylcellulose, 15.0%-25.0% of sodium carboxymethylcellulose and the like; the common components mainly comprise, by mass, 8.00%-12.0% of the blonanserin, 30.0%-50.0% of lactose and the like. By means of the tablet prepared from the sustained release preparation, the number of medicine taking can be decreased, the stable blood concentration can be maintained, and side effects can be reduced.
Owner:LIVZON PHARM GRP INC
Blonanserin tablet composition and preparation method thereof
ActiveCN107028903AAdvantages of chemical stabilityOrganic active ingredientsNervous disorderBlonanserinAdhesive
The invention relates to a blonanserin tablet composition and a preparation method thereof. Particularly, the blonanserin tablet composition comprises, by weight, 4 parts of blonanserin, 60-200 parts of diluting agents, 5-20 parts of disintegrating agents, 1-5 parts of adhesives, 0.1-2 parts of flow aids and 0.1-1 part of lubricants. The blonanserin tablet composition has excellent properties such as tablet dissolution performance stated in the specification.
Owner:SHENZHEN WANHE PHARMA
Process for preparing blonanserin
InactiveCN107397729AEliminate static electricityImprove liquidityOrganic active ingredientsNervous disorderAdhesiveDissolution
The invention discloses a preparation process of blonanserin tablets. The tablet contains blonanserin, hydroxypropyl cellulose (low substitution), lactose, hydroxypropyl cellulose, microcrystalline cellulose, magnesium stearate, silicon dioxide, and the preparation of the blonanserin tablet The process includes the following steps: 1) blonanserin raw material micropowder is premixed with silicon dioxide; 2) the premix is mixed with hydroxypropyl cellulose (low substitution) and lactose; 3) hydroxypropyl cellulose aqueous solution Make soft materials for binders, granulate, dry, and granulate; 4) Add silicon dioxide, microcrystalline cellulose and magnesium stearate, mix well, and compress into tablets. The blonanserin tablet prepared by the invention has the advantages of simple process operation, easy industrialization, good stability, good dissolution rate and the like.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
Separation method of blonanserin intermediate IV and other intermediates by high performance liquid chromatography
InactiveCN102297910BEfficient separationMake sure to dissolve completelyComponent separationFluid phaseBlonanserin
The invention discloses a separation method of a blonanserin intermediate IV and other intermediates by high performance liquid chromatography. The separation method selects octylsilane chemically bonded and silica as a chromatographic column of a filling agent (250mm multiplied by 4.6mm, 5mum), adopts a buffer solution and an organic modifier with different proportions as flowing phase, and the blonanserin intermediate IV and other intermediates which has the similar polarity with the blonanserin intermediate IV can be separated under the normal temperature by gradient elution, so that the accurate control of the quality of blonanserin can be realized.
Owner:江苏万全特创医药生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
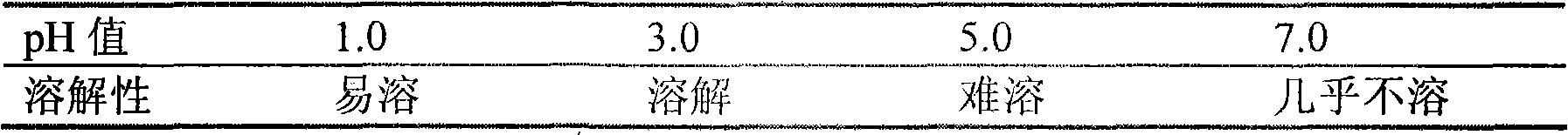



![2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine (blonanserin, blonanserin) and composition thereof 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine (blonanserin, blonanserin) and composition thereof](https://images-eureka.patsnap.com/patent_img/80081a78-03f1-40f0-a362-e83935b19185/A2009100695100011H1.PNG)
![2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine (blonanserin, blonanserin) and composition thereof 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine (blonanserin, blonanserin) and composition thereof](https://images-eureka.patsnap.com/patent_img/80081a78-03f1-40f0-a362-e83935b19185/A2009100695100011H2.PNG)
![2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine (blonanserin, blonanserin) and composition thereof 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine (blonanserin, blonanserin) and composition thereof](https://images-eureka.patsnap.com/patent_img/80081a78-03f1-40f0-a362-e83935b19185/A2009100695100012H1.PNG)