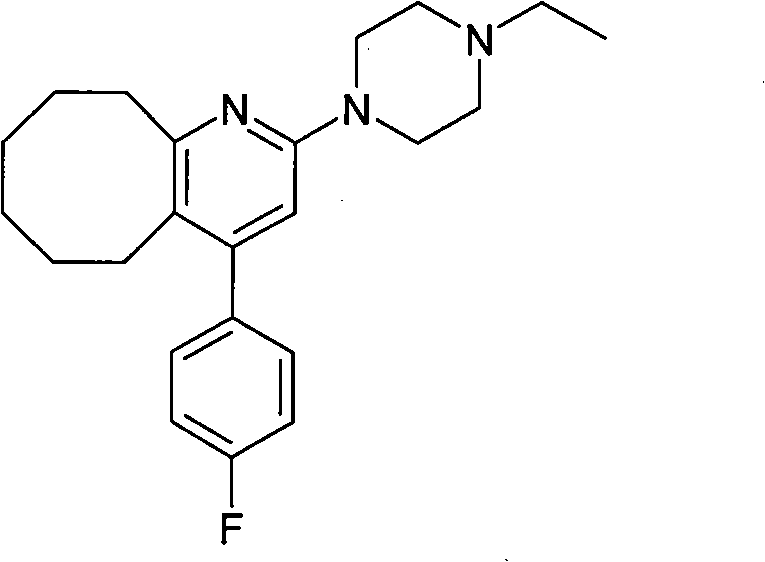
Crystal form B of blonanserin and preparing method thereof
A technology of crystal form and ethylpiperazine, which is applied in the field of blonanserin crystal form B and its preparation, can solve problems such as unsatisfactory stability, and achieve good development and utilization prospects, good stability, and difficulty in crystal transformation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
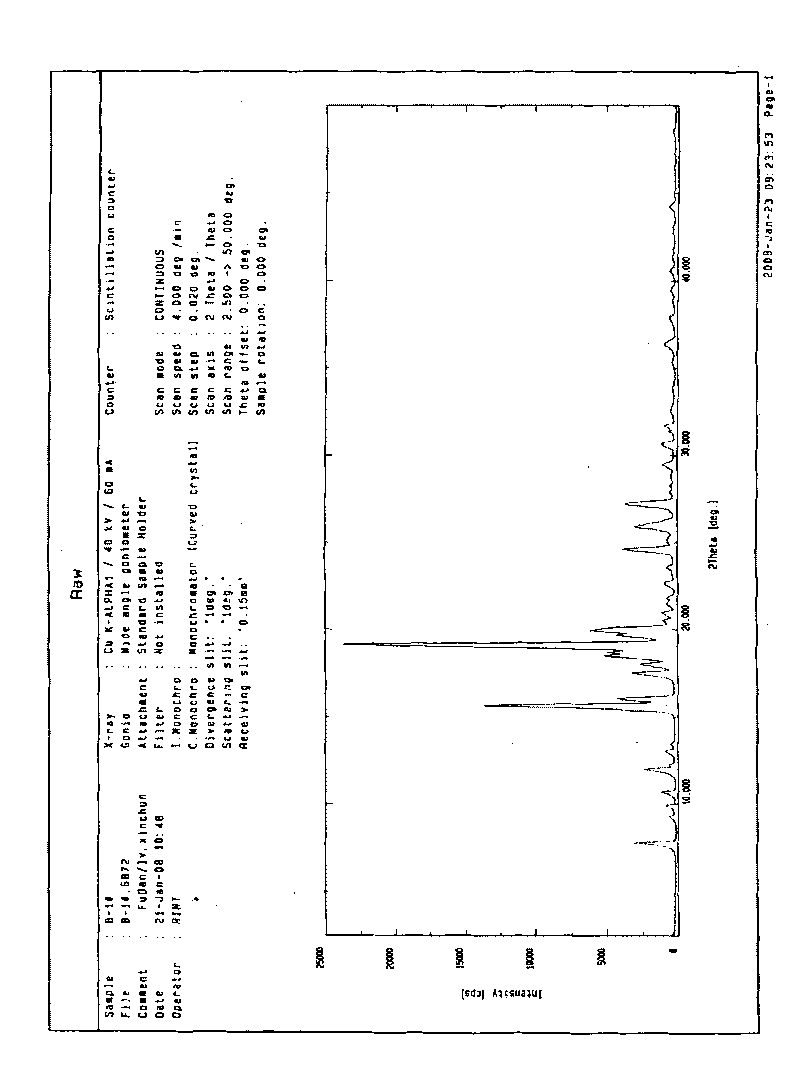
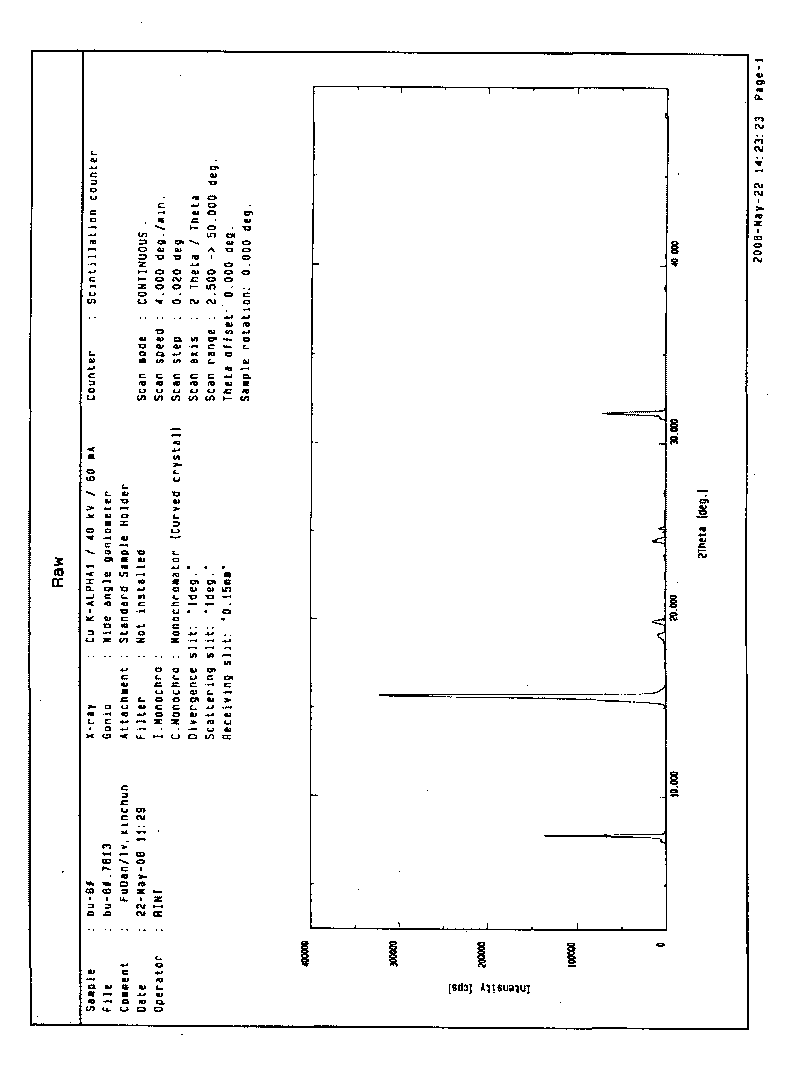
[0026] Add 1 g of blonanserin to 10 ml of ethanol and heat to dissolve at 78°C, then cool to room temperature while stirring, and filter after 2 hours. Dry at 60°C. The product was obtained 0.9 g. The product is proved by X-ray powder diffraction to be the crystal form B of 2-ethylpiperazine-4-(4-fluorobenzene)-5,6,7,8,9,10-hexahydrocyclooctanopyridine, It exhibits an X-ray powder diffraction pattern with characteristic peaks represented at about 7.74, 15.6, 19.1 and 31.64 in 2θ° as figure 2 shown. The melting point of the crystal form B was measured to be 125-127°C.
Embodiment 2
[0028] Add 7.4 g of blonanserin to 100 ml of ethanol and heat to dissolve at 78°C, then cool to room temperature while stirring, and filter after 2 hours. Dry at 60°C. 6.5 g of product were obtained. The product was proved to be crystal form B of 2-ethylpiperazine-4-(4-fluorobenzene)-5,6,7,8,9,10-hexahydrocyclooctanopyridine by X-ray powder diffraction. The melting point of the crystal form B was measured to be 125-126°C.
Embodiment 3
[0030] Add 82.8 g of blonanserin to 500 ml of ethanol and heat to dissolve at 78°C, then cool to room temperature while stirring, and filter after 2 hours. Dry at 60°C. 72 g of product were obtained. The product was proved to be crystal form B of 2-ethylpiperazine-4-(4-fluorobenzene)-5,6,7,8,9,10-hexahydrocyclooctanopyridine by X-ray powder diffraction. The melting point of the crystal form B was measured to be 125-126°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com