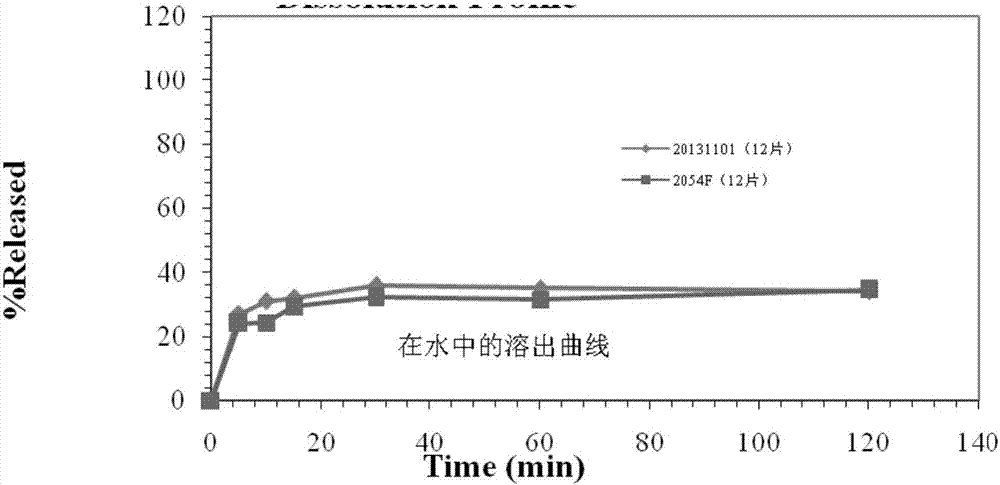
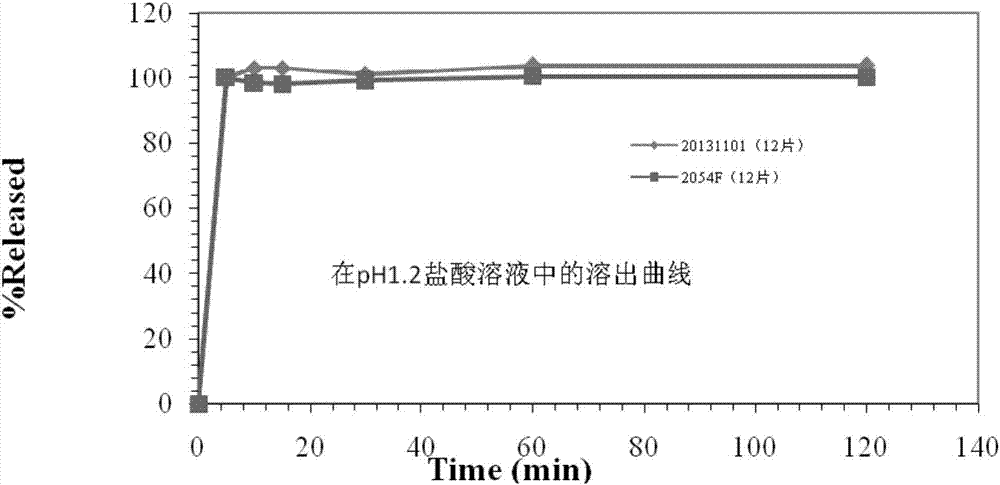
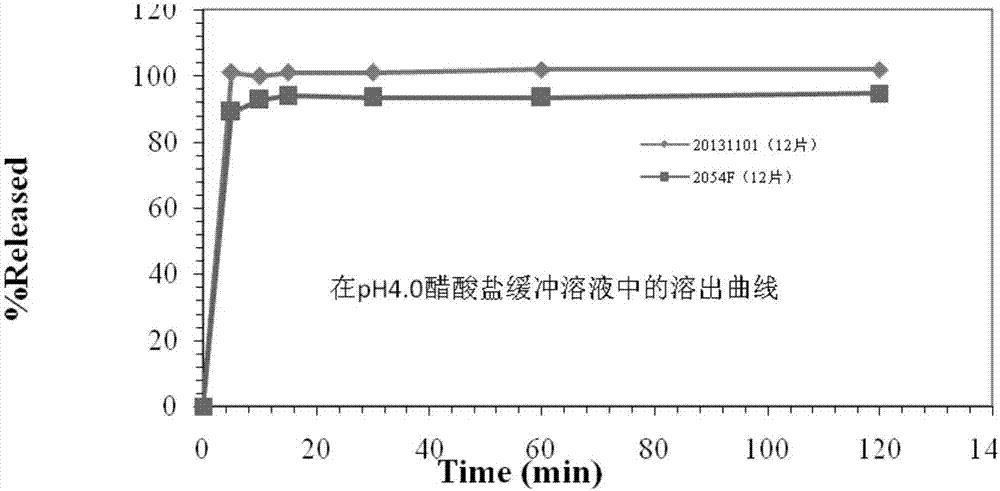
Blonanserin tablet composition and preparation method thereof
A technology for blonanserin and composition, which is applied in the field of medicine and can solve the problems of large loss of active ingredients, insufficient viscosity of adhesives, small hardness of tablets and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: blonanserin tablets
[0081] prescription:
[0082] Blonanserin (the main drug) 4.0 parts by weight, lactose (diluent) 60 parts by weight, Microcrystalline Cellulose (Thinner) 45 parts by weight, Hydroxypropyl Cellulose (Binder) 2 parts by weight, Low-substituted hydroxypropyl cellulose (disintegrant) 8 parts by weight, Silicon dioxide (glidant) 0.6 parts by weight, Magnesium Stearate (Lubricant) 0.3 parts by weight, Purified water (binding solvent) Appropriate amount (solvent used in the process and eventually removed).
[0083] Preparation method:
[0084] (i) Pre-crushing various raw materials and auxiliary materials to a fine powder that can pass through 120 mesh;
[0085] (ii) Micronize blonanserin and 1.5 times its weight of microcrystalline cellulose until the particle size is less than 10 μm (more than 90% of the particle diameter is within the range of 3 to 10 μm), and then add ha...
Embodiment 2
[0088] Embodiment 2: blonanserin tablets
[0089] prescription:
[0090]
[0091]
[0092] Preparation method:
[0093] (i) Pre-crushing various raw materials and auxiliary materials to a fine powder that can pass through 120 mesh;
[0094] (ii) Micronize blonanserin and microcrystalline cellulose with 1 times its weight until the particle size is less than 10 μm (more than 90% of the particle diameter is within the range of 3 to 10 μm), and then add half the amount of the prescription Mix disintegrants and continue to crush until the particle size of all particles is less than 25 μm to obtain micronized powder;
[0095] (iii) Mix the micronized powder obtained in step (ii) with the remaining amount of diluent, the remaining amount of disintegrant and half the amount of glidant in the prescription, put it in a fluidized bed granulator, and prepare the binder with water into a binder solution with a concentration of 3%, and use it to carry out fluidized bed granulati...
Embodiment 3
[0097] Embodiment 3: blonanserin tablets
[0098] prescription:
[0099] Blonanserin (the main drug) 4.0 parts by weight, lactose (diluent) 67 parts by weight, Microcrystalline Cellulose (Thinner) 133 parts by weight, Hydroxypropyl Cellulose (Binder) 5 parts by weight, Low-substituted hydroxypropyl cellulose (disintegrant) 20 parts by weight, Silicon dioxide (glidant) 2 parts by weight, Magnesium Stearate (Lubricant) 0.1 parts by weight, Purified water (binding solvent) Appropriate amount (solvent used in the process and eventually removed).
[0100] Preparation method:
[0101] (i) Pre-crushing various raw materials and auxiliary materials to a fine powder that can pass through 120 mesh;
[0102] (ii) Micronize blonanserin and microcrystalline cellulose twice its weight until the particle size is less than 10 μm (more than 90% of the particle diameter is within the range of 3 to 10 μm), and then add half the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com