Blonanserin tablet and preparation method thereof
A technique for blonanserin, tablet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
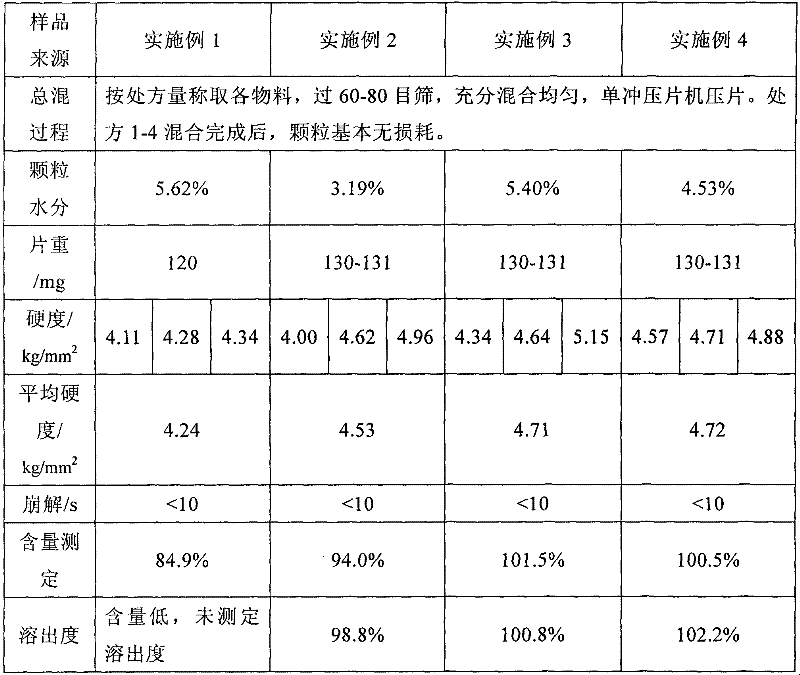
Examples
Embodiment 1
[0020] Compatibility test of raw materials and auxiliary materials: investigate the stability of blonanserin and various direct pressure auxiliary materials after 10 days of accelerated test at 60°C and RH75%.
[0021] Sample treatment: Transfer the sample with the composition shown in Table 1 to a 25ml volumetric flask, dilute to the mark with mobile phase, shake well, and detect with high performance liquid phase.
[0022] Table 1 Compatibility test results
[0023]
[0024] It can be seen from the data in Table 1 that the blonanserin raw material itself is very stable, and when it is compatible with other direct-pressing auxiliary materials, the content does not drop significantly after being inspected under certain temperature and humidity conditions, which proves that they can be mixed and pressed. sliced.
Embodiment 2
[0026] Prescription 1
[0027] Blonanserin 4.0g
[0028] Cellulose-Lactose 110.0g
[0029] Croscarmellose Sodium 4.0g
[0030] Magnesium Stearate 1.0g
[0031]
[0032] 119g, made into 1000 pieces
[0033] Preparation method: Weigh each component according to the prescription amount, pass through a 70-mesh sieve, mix well, granulate, and tablet with a single-punch tablet machine.
Embodiment 3
[0035] Prescription 2
[0036] Blonanserin 4.0g
[0037] Spray dried lactose 90.0g
[0038] Microcrystalline Cellulose 102 30.0g
[0039] Croscarmellose Sodium 4.0g
[0040] Magnesium Stearate 1.0g
[0041]
[0042] 129g, made into 1000 pieces
[0043] Preparation method: Weigh each component according to the prescription amount, pass through a 60-mesh sieve, mix well, granulate, and tablet with a single-punch tablet machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com