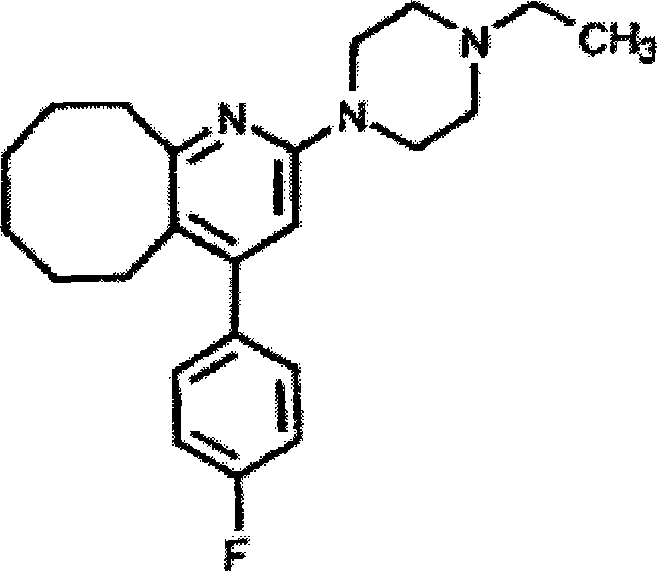
Pharmaceutical composition of blonanserin and preparation method thereof
A technology for compositions and mixtures, which is applied in the directions of drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, and can solve the problems of inability to obtain preparation products and inability to meet industrialization requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Preparation Process
[0025] Blonanserin is crushed through a 100-mesh sieve, mannitol, lactose, calcium hydrogen phosphate, and microcrystalline cellulose are respectively passed through a 80-mesh sieve, and magnesium stearate is passed through a 60-mesh sieve. Mix in a fast stirring granulator according to the prescription amount, add water-based soft material, pass through a 18-mesh sieve, dry in an oven at 55°C, pass through a 24-mesh sieve for granulation, add the prescription amount of magnesium stearate, and mix evenly Tablets, each containing 5mg of blonanserin.
Embodiment 2
[0027]
[0028] Preparation Process
[0029] Blonanserin is crushed through a 100-mesh sieve, mannitol, lactose, calcium hydrogen phosphate, and microcrystalline cellulose are respectively passed through a 80-mesh sieve, mixed in a fast stirring granulator according to the prescribed amount, added with water-based soft materials, and passed through a 18-mesh sieve Sieve, after drying in an oven at 55°C, pass through a 24-mesh sieve for granulation to obtain granules with uniform and full color, add the prescribed amount of micro-powder silica gel, mix well, and put them into capsule shells. Each capsule contains 5mg of blonanserin.
Embodiment 3
[0031]
[0032] Preparation Process
[0033] Blonanserin was crushed through a 100-mesh sieve, mannitol, lactose, calcium hydrogen phosphate, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose were respectively passed through a 80-mesh sieve, mixed in a fast stirring granulator according to the prescribed amount, added water The soft material is passed through a 18-mesh sieve, dried in an oven at 55°C, passed through a 24-mesh sieve for granulation, added the prescribed amount of magnesium stearate, mixed evenly, and pressed into tablets. Each capsule contains blonanserin 10mg
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com