Sustained release preparation of blonanserin and preparation method thereof
A blonanserin and slow-release technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as inconvenient use, side effects, "peaks and valleys", and achieve price Inexpensive, easy to take, and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
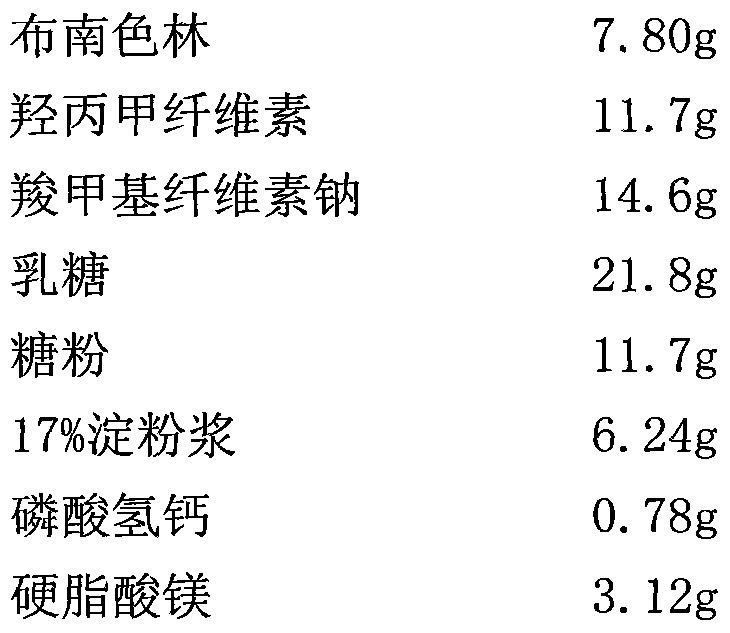
[0021] (1) Prescription of sustained-release blonanserin pellets
[0022]
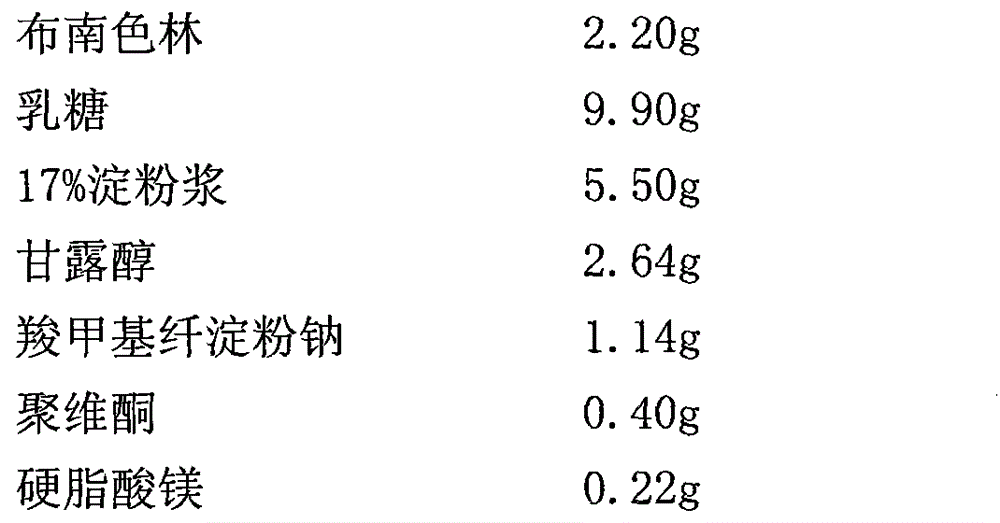
[0023] (2) Ordinary blonanserin pellet prescription
[0024]
[0025] Prepare the tablet mix recipe
[0026] component name
[0027] Compressed into 280 tablets.
[0028] According to the specific preparation method of the above prescription:
[0029] 1), based on the mass percentage of slow-release blonanserin components, weigh blonanserin, hydroxypropylmethylcellulose, sodium carboxymethylcellulose, lactose, sugar powder, 17% starch slurry, and mix well , then add the prescribed amount of calcium hydrogen phosphate and magnesium stearate, continue to mix evenly, and add an appropriate amount of water to prepare a soft material;
[0030] 2), according to the mass percentage of ordinary granule blonanserin, weigh blonanserin, lactose, 17% starch slurry, mannitol, povidone, sodium carboxymethyl starch, and magnesium stearate. , adding blonanserin sustained-release granules, mixing un...
Embodiment 2
[0035] Prepare according to the prescription of embodiment 1 slow-release component and common component prescription, prepare the mixed prescription quantity of tablet
[0036] component name
Dosage (g)
Sustained-release blonanserin pellets
75.00
Ordinary blonanserin pellets
25.00
Total
100.00
[0037] Compressed into 280 tablets.
[0038] According to the specific preparation method of the above prescription:
[0039] 1), based on the mass percentage of slow-release blonanserin components, weigh blonanserin, hydroxypropylmethylcellulose, sodium carboxymethylcellulose, lactose, sugar powder, 17% starch slurry, and mix well , then add the prescribed amount of calcium hydrogen phosphate and magnesium stearate, continue to mix evenly, and add an appropriate amount of water to prepare a soft material;
[0040]2), according to the mass percentage of ordinary granule blonanserin, weigh blonanserin, lactose, 17% starch slurry, m...
Embodiment 3
[0042] Prepare according to the prescription of embodiment 1 slow-release component and common component prescription, prepare the mixed prescription quantity of tablet
[0043] component name
Dosage (g)
Sustained-release blonanserin pellets
80.00
Ordinary blonanserin pellets
20.00
Total
100.00
[0044] Compressed into 280 tablets.
[0045] According to the specific preparation method of the above prescription:
[0046] 1), based on the mass percentage of slow-release blonanserin components, weigh blonanserin, hydroxypropylmethylcellulose, sodium carboxymethylcellulose, lactose, sugar powder, 17% starch slurry, and mix well , then add the prescribed amount of calcium hydrogen phosphate and magnesium stearate, continue to mix evenly, and add an appropriate amount of water to prepare a soft material;
[0047] 2), according to the mass percentage of ordinary granule blonanserin, weigh blonanserin, lactose, 17% starch slurry, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com