New preparation method of Blonanserin intermediate
A technology for blonanserin and intermediates, which is applied in the new field of preparation of blonanserin intermediates, can solve problems such as complicated operations and complex operations, and achieve the effects of simple post-processing, simple reaction steps, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
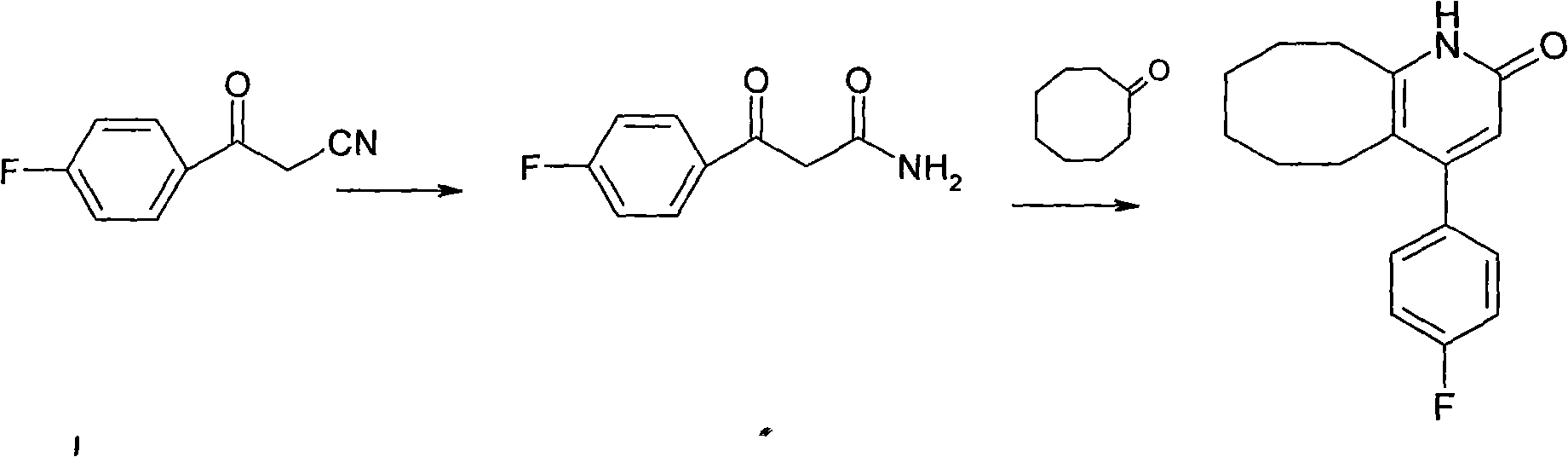
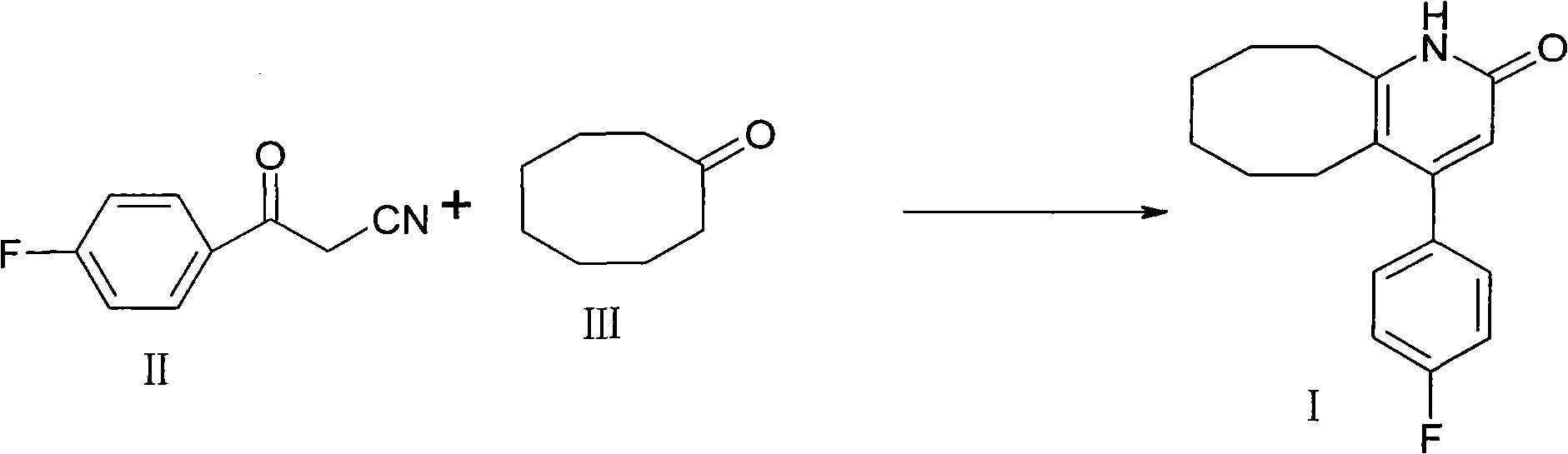
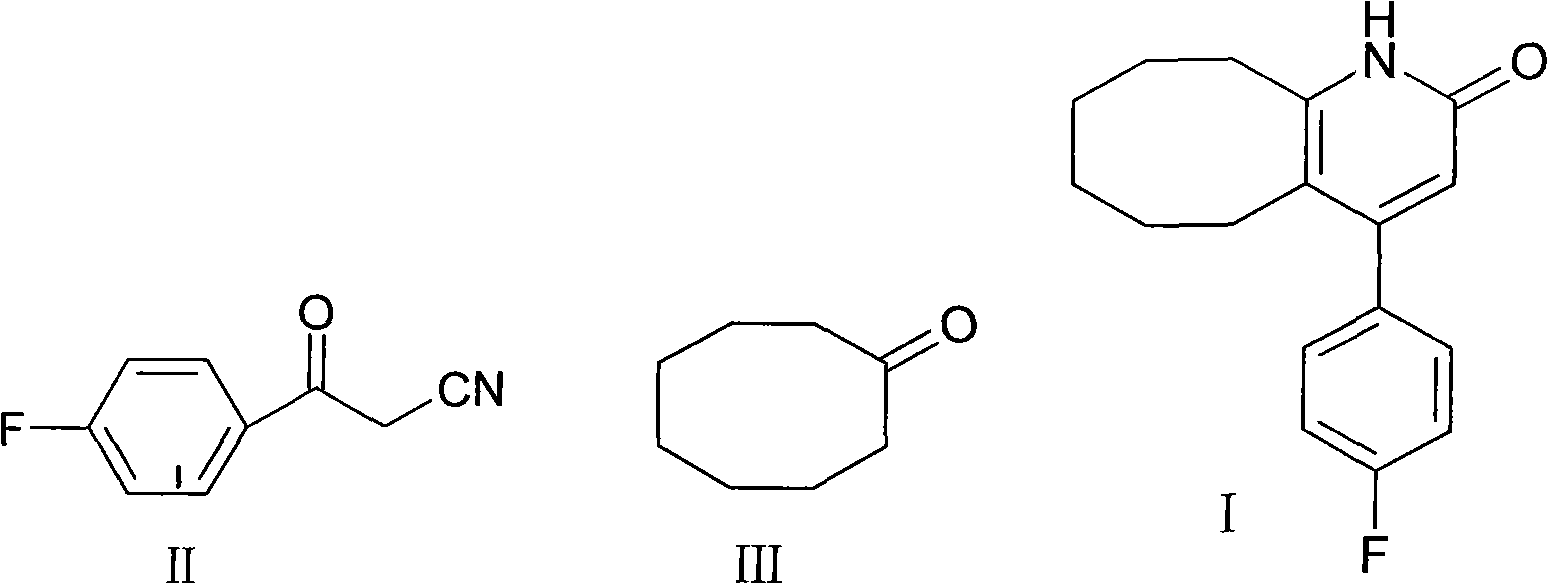
[0020] Put 100ml of toluene, 5g of p-fluorobenzoylacetonitrile (II), 5g of cyclooctanone (III), 5g of p-toluenesulfonic acid, and 5ml of concentrated sulfuric acid into the reaction flask. Heat and stir at about 110°C and reflux for 3 hours. After the central control reaction is complete After adding water to wash, extract and separate the layers, the organic layer is decolorized and concentrated to obtain 6.8g crystals. The yield is 82%, the purity is 98.4%, and the melting point is 236-238°C.
Embodiment 2
[0022] Put 150ml of toluene, 10g of p-toluenesulfonic acid into the reaction flask, heat and stir, continue to add 5g of p-fluorobenzoylacetonitrile, 5g of cyclooctanone and 5ml of concentrated sulfuric acid, reflux for 3 hours at about 110℃, add water after the control reaction is complete Washing, extracting and separating, the organic layer is decolorized and concentrated to obtain 7 g of crystals. The yield is 85%, the purity is 98.5%, and the melting point is 236-238°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com