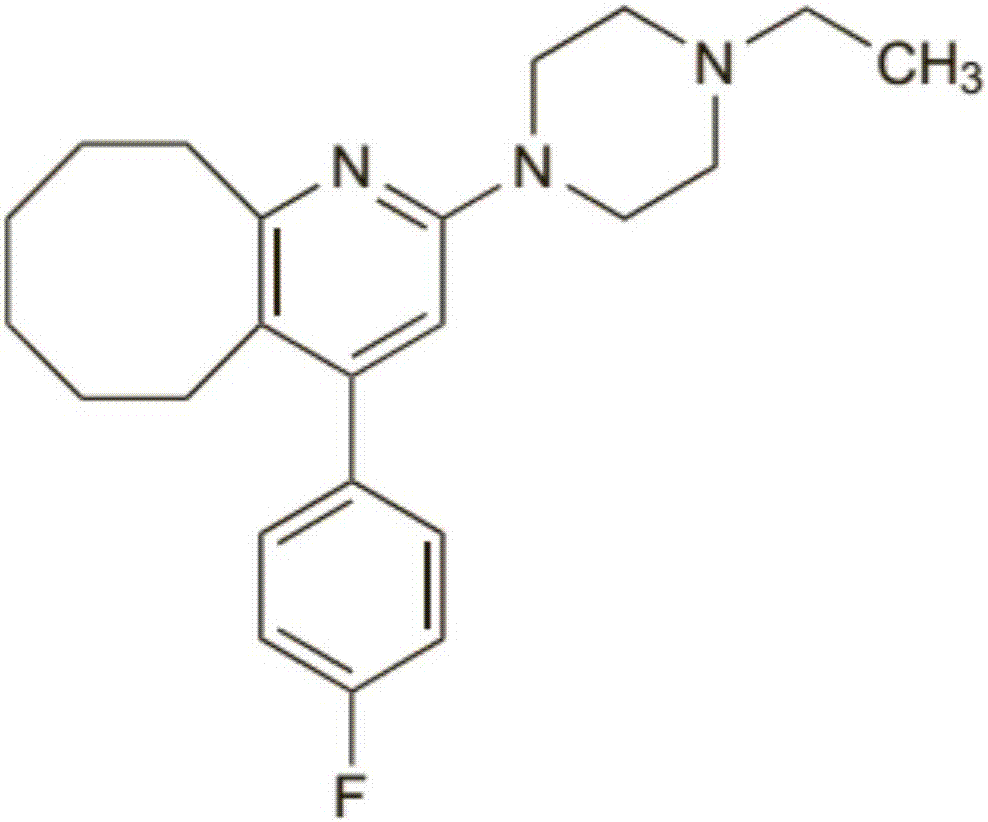
Blonanserin and preparation method thereof
A technology of blonanserin and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Preparation of Blonanserin (#206E57)
[0089] step 1):
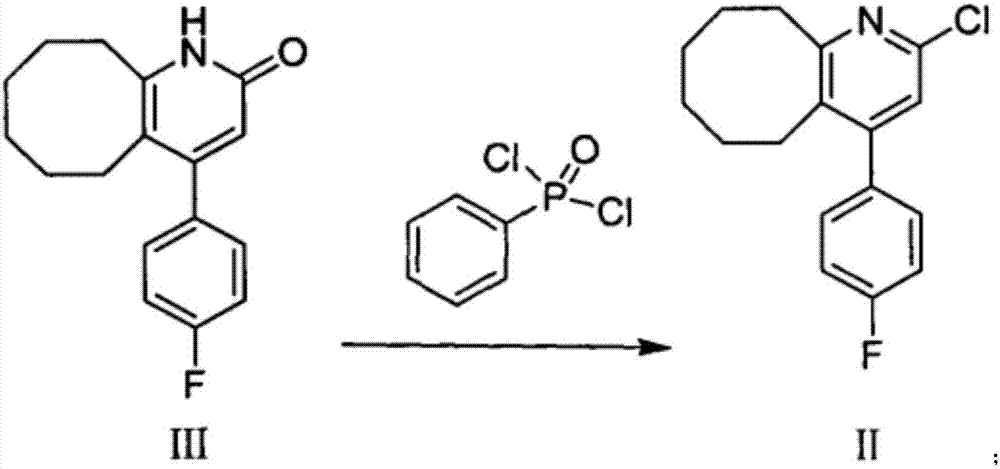
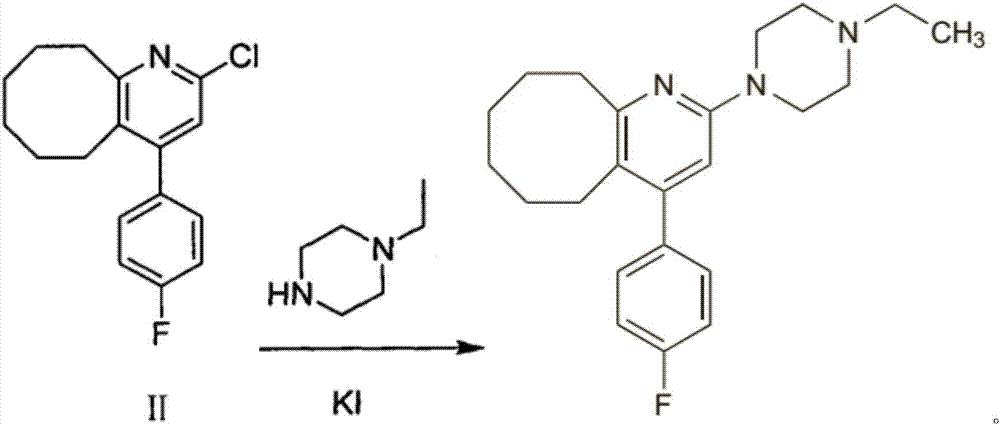
[0090] Add 1180ml of phenylphosphonic dichloride into the reactor, cool to below 25°C, add 900g of compound (III) into the reactor in batches at 40°C, after the addition is complete, heat the system to an internal temperature of 165-170°C, Continue the heat preservation reaction. After the raw materials are completely reacted, the temperature of the system is lowered to about 40-45°C. Slowly add the reaction solution into 13.5L of dichloromethane, stir, and then slowly add the reaction system into 13.5L of ice water, keeping the temperature below 5°C. , slowly add 10% NaOH solution dropwise to adjust the pH value to 9-10, add diatomaceous earth, filter, separate liquids, combine the organic phases, dry, remove dichloromethane, obtain about 933g dark brown solid, add 465ml absolute ethanol After refining, 631 g of an earthy yellow solid product, namely the compound of formula (II), was obtained with a...
Embodiment 2
[0093] Example 2: Preparation of Blonanserin (#206E68)
[0094] step 1):
[0095] Add 1180ml of phenylphosphonic dichloride into the reactor, cool to below 25°C, add 900g of compound (III) into the reactor in batches at 40°C, after the addition is complete, heat the system to an internal temperature of 165-170°C, Continue the heat preservation reaction. After the raw materials are completely reacted, the temperature of the system is lowered to about 40-45°C. Slowly add the reaction solution into 13.5L of dichloromethane, stir, and then slowly add the reaction system into 13.5L of ice water, keeping the temperature below 5°C. , slowly add 10% NaOH solution dropwise to adjust the pH value to 9-10, add diatomaceous earth, filter, separate liquids, combine the organic phases, dry, remove dichloromethane to obtain about 928g dark brown solid, add 900ml isopropanol After refining, 550.2 g of an earthy yellow solid, namely the compound of formula (II), was obtained with a purity g...
Embodiment 3
[0098] Embodiment 3: Preparation of blonanserin (E1)
[0099] step 1):
[0100] Add 1150ml of phenylphosphonic dichloride into the reactor, cool to below 25°C, add 900g of compound (III) into the reactor in batches at 40°C, after the addition is complete, heat the system to an internal temperature of 165-170°C, Continue the heat preservation reaction. After the reaction of the raw materials is complete, the temperature of the system is lowered to about 40-45°C. Slowly add the reaction solution into 13L of dichloroethane, stir, and then slowly add the reaction system into 13L of ice water, keeping the temperature below 5°C. Slowly add 10% NaOH solution dropwise to adjust the pH value to 9-10, add diatomaceous earth, filter, separate the liquids, combine the organic phases, dry, and remove dichloromethane to obtain about 930 g of dark brown solids, which are refined by adding 465 ml of isopropanol , to obtain 632 g of an earthy yellow solid product, namely the compound of for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com