Blonanserin dropping pill and preparation method thereof
A technology of blonanserin and Lin drop pills, which is applied in pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as non-acknowledgment of illness, lack of self-awareness, poor compliance with medication, etc., to achieve The effect of simple prescription, simple equipment and simple composition of prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Prescription 1000 capsules
[0040] Blonanserin 4.0g
[0041] Macrogol 6000 40.0g
[0042] Crospovidone 4.0g
[0043] Tween-80 0.5g
[0044] Carmine 0.5g
[0045]Preparation process: Weigh blonanserin and polyethylene glycol 6000 and mix evenly, heat to 70°C, stir and melt to dissolve the drug completely; add other excipients in the prescription, and mix well. Under the condition of heat preservation at 70°C, use a dropper with an inner diameter of 2.5mm to drop the mixed solution into liquid paraffin at 0°C, filter, wash, dry, and pack after solidification.
[0046] Each pill weighs 49mg and contains 4.0mg of blonanserin.
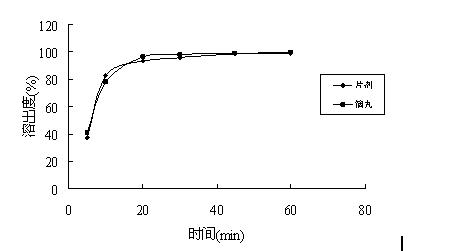
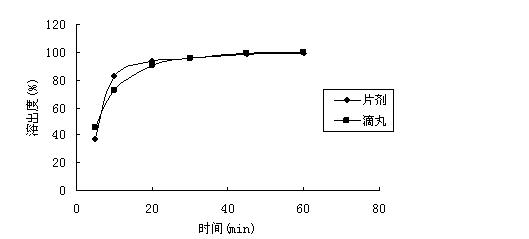
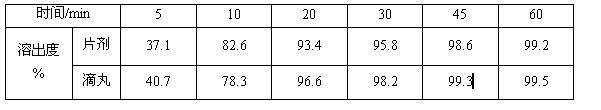
[0047] (1) Dissolution test: Dissolution test was carried out between the prepared dropping pills and foreign marketed tablets.
[0048] Test method: Chinese Pharmacopoeia 2010 edition two appendix XC second method, 50rpm;
[0049] Dissolution medium: pH6.0 phosphate buffer;
[0050] Control preparation: blonanserin tablets (Dainippon Sumito...
Embodiment 2
[0062] Prescription 1000 capsules
[0063] Blonanserin 1.0g
[0064] Polyethylene glycol 6000 25.0g
[0065] Microcrystalline Cellulose 6.0g
[0066] Sodium Lauryl Sulfate 0.3g
[0067] Carmine 0.3g
[0068] Sucralose 0.3g
[0069] Preparation process: with embodiment 1.
Embodiment 3
[0071] Prescription 1000 capsules
[0072] Blonanserin 10.0g
[0073] Macrogol 4000 50.0g
[0074] Crospovidone 4.0g
[0075] Tween-80 1.2g
[0076] Carmine 0.5g
[0077] Xylitol 0.5g
[0078] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com