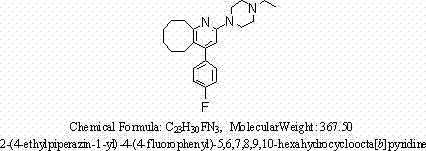
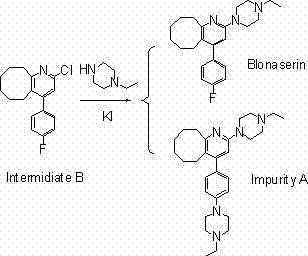
Method for synthesizing Blonanserin
A synthetic method, the technology of blonanserin, applied in the direction of organic chemistry, can solve the problems of many side reactions, high pollution, strong irritation of chlorinated reagents, etc., and achieve simple and easy to control operation, low reaction temperature, and product purity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 2-p-toluenesulfonic acid-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydroaromatic octa[b]pyridine ester (intermediate II-1 ) preparation
[0034] Under stirring, dissolve 2.71g (10.0 mmol) of intermediate I in 40 mL of chloroform, add 1.93 g (10.2 mmol) of p-toluenesulfonyl chloride in 5 mL of chloroform, drop in 1.4 mL of pyridine, and stir at 40 °C Reaction, TLC monitors the completion of the reaction, pours the reactant into 100mL of water with stirring, adjusts the pH to neutral, adds methyl tert-butyl ether for extraction, separates the liquid, washes the extract with water, and removes the extract by rotary evaporation to obtain intermediate II-1 White solid, the crude product was recrystallized to obtain 3.18g of intermediate II-1, yield 75.3%, m.p.: 117~118 ℃.
[0035]
[0036] The structure of intermediate Ⅱ-1 is:
[0037] MS: m / e 426,[M+H]
[0038] IR absorption peak (cm -1 ): 2958.5, 29299, 2855.2, 1606.9, 1592.6, 1549.1, 1509.8, 1470, 1447.1, 1370....
Embodiment 2
[0040] Example 2 2-p-toluenesulfonic acid-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydroaromatic octa[b]pyridine ester (intermediate II-1 ) preparation
[0041] According to the method of Example 1, the number of moles of p-toluenesulfonyl chloride was adjusted to be twice that of the reactant intermediate I, and the yield of intermediate II-1 was 83.5%.
Embodiment 3
[0042] Example 3 2-p-toluenesulfonic acid-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydroaromatic octa[b]pyridine ester (intermediate II-1 ) preparation
[0043] According to the method of embodiment 1, the molar number of p-toluenesulfonyl chloride is adjusted to be 5 times, 10 times, 20 times of reactant intermediate I, and the yield of intermediate II-1 is all lower than 75%. Dealing with large losses.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com