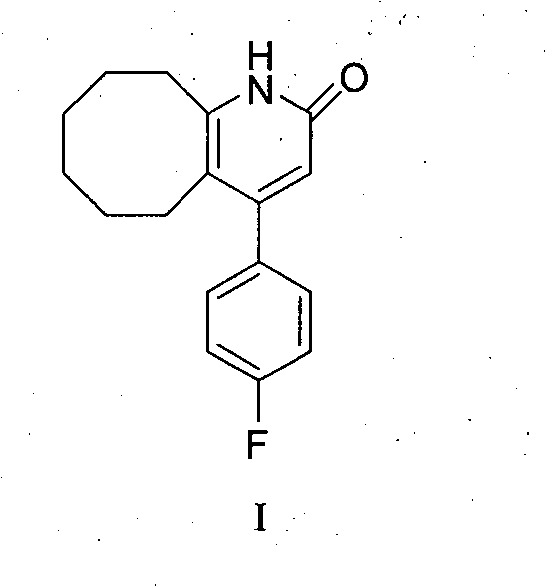
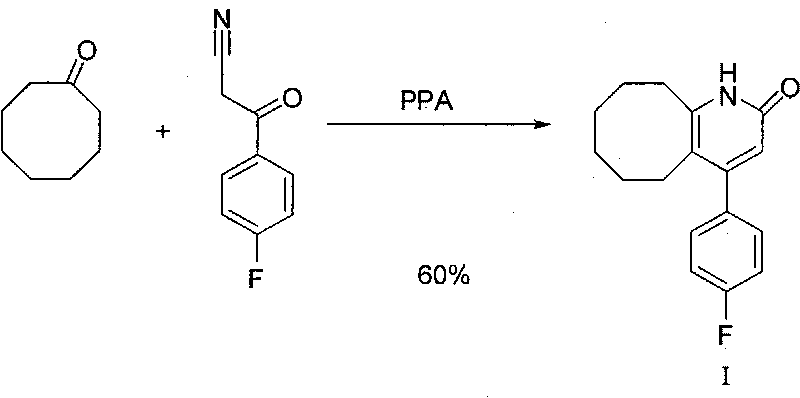
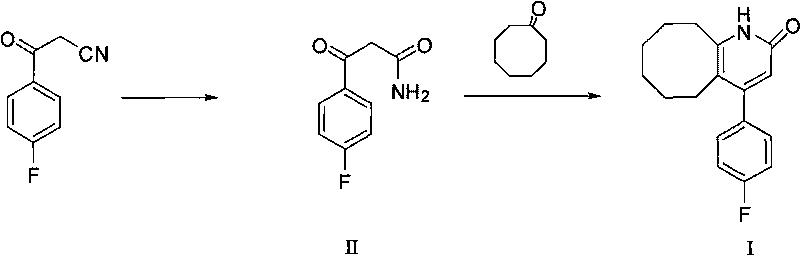
Preparing method of blonanserin intermediate
A technology of blonanserin and intermediate, which is applied in the field of medicinal chemistry to achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of 3-(4-fluorophenyl)-3 oxopropionamide (II):
[0027] In a 250mL reaction flask, add 26g of polyphosphoric acid solution to 2g of 3-(4-fluorophenyl)-3-oxopropionitrile. React at 75°C for 2 hours, TLC shows that the reaction is almost complete, add 60ml of ice water, stir for 1 hour, add KOH to adjust the pH value, extract, dry, concentrate and dry to obtain 2g of white solid (II). Yield 90%. (MS: m / z 180.04 (M-H+))
[0028] Preparation of 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocyclooctanopyridin-2(1H)-one (I):
[0029] Add (II) 0.5g, p-toluenesulfonic acid 1.0g, cyclooctanone 0.4g and toluene 50ml into a 250ml reaction flask, heat to 110°C for 3 hours, then concentrate the reaction solution to dryness, add dichloromethane 50ml was stirred for 10 minutes, and washed with water until neutral. Drying, concentration and drying gave 0.7 g of white solid (I), with a yield of 93%.
[0030] (Anal. Calcd. C, 75.25; H, 6.69; N, 5.16. Found: C, 70.52; H, 6....
Embodiment 2
[0032] Example 2: Preparation of 3-(4-fluorophenyl)-3 oxopropionamide (II):
[0033] In a 250mL reaction flask, add 80g of polyphosphoric acid solution to 5g of 3-(4-fluorophenyl)-3-oxopropionitrile. Reacted at 75°C for 5 hours, TLC showed that the reaction was almost complete, added 150ml of ice water, stirred for 1 hour, extracted with ethyl acetate, washed with saturated sodium bicarbonate, dried, concentrated and dried to obtain 4.8g of white solid (II). Yield 86.4%.
[0034] Preparation of 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocyclooctanopyridin-2(1H)-one (I):
[0035] Add 2g of (II), 5g of p-toluenesulfonic acid, 1.6g of cyclooctanone and 200ml of toluene into a 500mL reaction flask, heat to 110°C and react for 3.5 hours, then concentrate the reaction solution to dryness, add 300ml of dichloromethane and stir 10 minutes, add water to wash until neutral. Drying, concentration and drying gave 2.5 g of white solid (I). Yield: 83.0%.
[0036] Two-step total yield: 71...
Embodiment 3
[0038] The preparation of 3-(4-fluorophenyl)-3 oxopropionamide (II):
[0039] In a 1L reaction flask, 308 g of polyphosphoric acid solution was added to 29.5 g of 3-(4-fluorophenyl)-3-oxopropionitrile. Reacted at 75°C for 5 hours, TLC showed that the reaction was almost complete, added 600ml of ice water, stirred for 1 hour, extracted with ethyl acetate, washed with saturated sodium bicarbonate, dried, concentrated and dried to obtain 27.4g of white solid (II). Yield 83.6%.
[0040] Preparation of 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocyclooctanopyridin-2(1H)-one (I):
[0041] Add (II) 18.5g, p-toluenesulfonic acid 35g, cyclooctanone 12.9g and toluene 500ml into a 1L reaction flask, heat to 110°C for 5 hours, then concentrate the reaction solution to dryness, add 300ml of dichloromethane Stir for 10 minutes, add water to wash until neutral. Drying, concentration and drying gave 14 g of white solid (I). Yield: 76%.
[0042] Two-step total yield: 63.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com