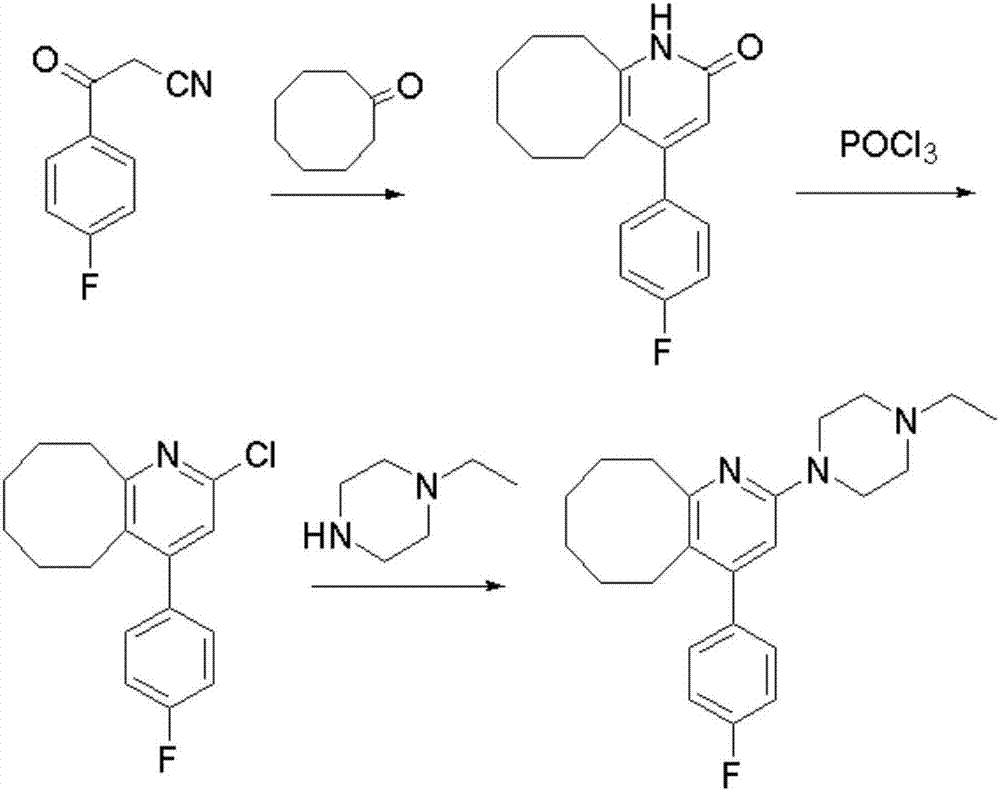
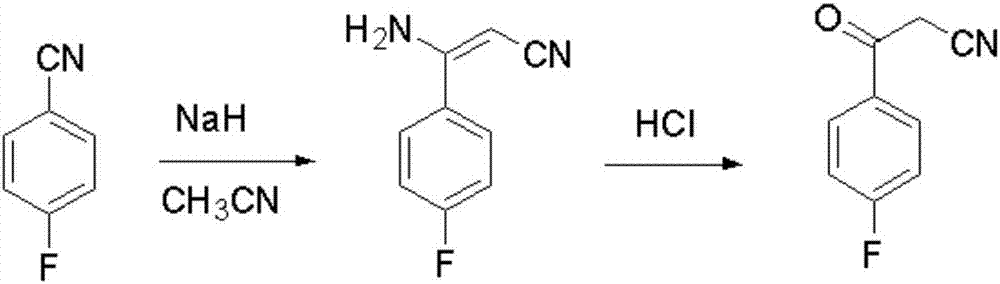
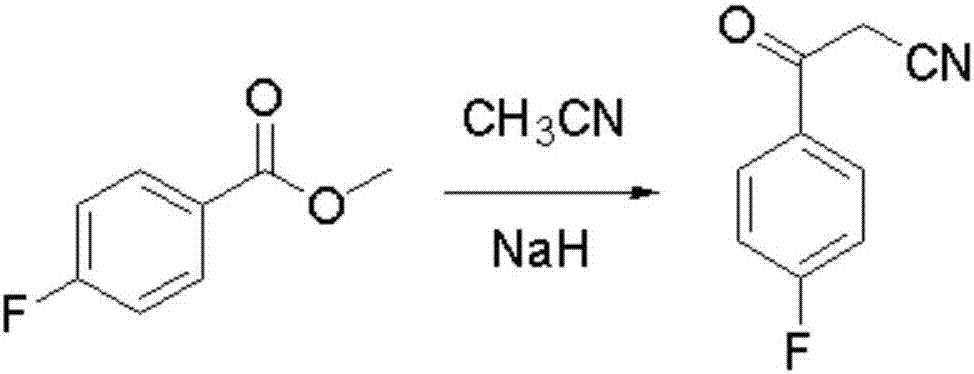
Blonanserin intermediate 4-fluorobenzoylacetonitrile synthesis method
A technology of fluorobenzoylacetonitrile and p-fluorobenzonitrile, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as production safety hazards, difficulty in removal, and flammability in contact with water, and achieve improved safety, stable yield, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 93.5g of potassium tert-butoxide and 450mL of isopropyl ether into the three-necked flask, and dropwise add a mixed solution composed of 34g of acetonitrile / 4g of tert-butanol / 200mL of isopropyl ether / 50g of p-fluorobenzonitrile under stirring. The temperature of the process reaction system is controlled within the range of 10-20°C. After the dropwise addition, the temperature of the reaction system was maintained in the range of 30-32° C. for 18 hours.
[0029] After the basic reaction of the raw materials was detected by TLC, 200 mL of water was added dropwise to quench the reaction, and the temperature of the reaction system was controlled below 30° C. during the dropwise addition. Then the isopropyl ether was distilled off under reduced pressure at room temperature, and then 300 mL of dichloromethane was added to dissolve and the layers were separated. The aqueous layer was extracted with dichloromethane (150 mL×2), and the organic phases were combined to prepa...
Embodiment 2
[0032] Add 205.7g of potassium tert-butoxide and 900mL of isopropyl ether into the three-necked flask, and add dropwise the mixed solution made of 68g of acetonitrile / 8g of tert-butanol / 400mL of isopropyl ether / 100g of p-fluorobenzonitrile under stirring. The process temperature is controlled within the range of 10°C to 25°C. After the dropwise addition, the temperature of the reaction system was maintained in the range of 28° C. to 30° C. for 20 hours.
[0033] After the basic reaction of the raw materials was detected by TLC, 400 mL of water was added to quench the reaction, and the temperature during the quenching process was controlled below 30°C. Then distill off isopropyl ether under reduced pressure at room temperature, then add 600mL dichloromethane to dissolve and separate the layers, the aqueous layer is extracted with dichloromethane (300mL×2), and the organic phases are combined to prepare for the next step of reaction.
[0034] Add 560 mL of 3 mol / L hydrochloric ...
Embodiment 3
[0036] Add 187.0 g of potassium tert-butoxide and 900 mL of tetrahydrofuran into the three-necked flask, and add dropwise a mixed solution composed of 68 g of acetonitrile / 8 g of tert-butanol / 400 mL of isopropyl ether / 100 g of p-fluorobenzonitrile under stirring, and the temperature of the dropping process is controlled In the range of 10°C to 25°C. After the dropwise addition, the temperature of the reaction system was maintained in the range of 30° C. to 32° C. for 21 hours.
[0037] After the basic reaction of the raw materials was detected by TLC, 400 mL of water was added to quench the reaction, and the temperature during the quenching process was controlled below 30°C. Then distill off isopropyl ether under reduced pressure at room temperature, then add 600mL dichloromethane to extract and separate layers, and then extract the aqueous layer with dichloromethane (300mL×2), and combine the organic phases to prepare for the next step of reaction.
[0038] Add 560 mL of 3 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com