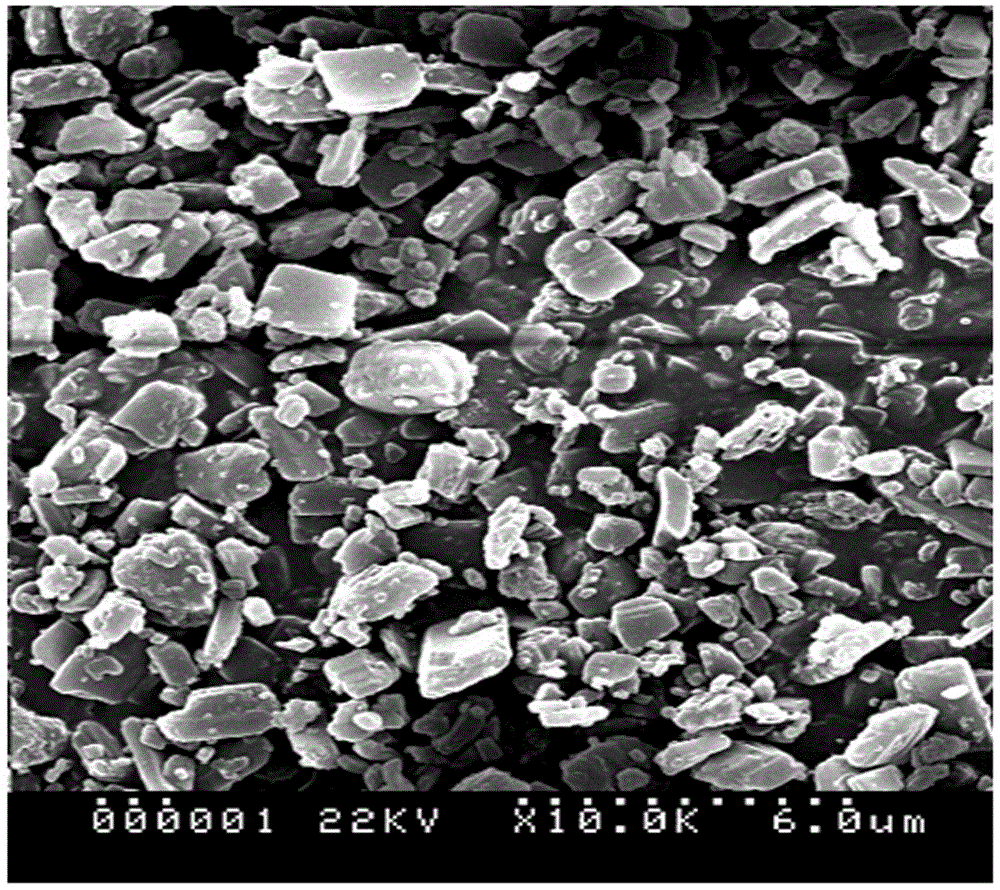
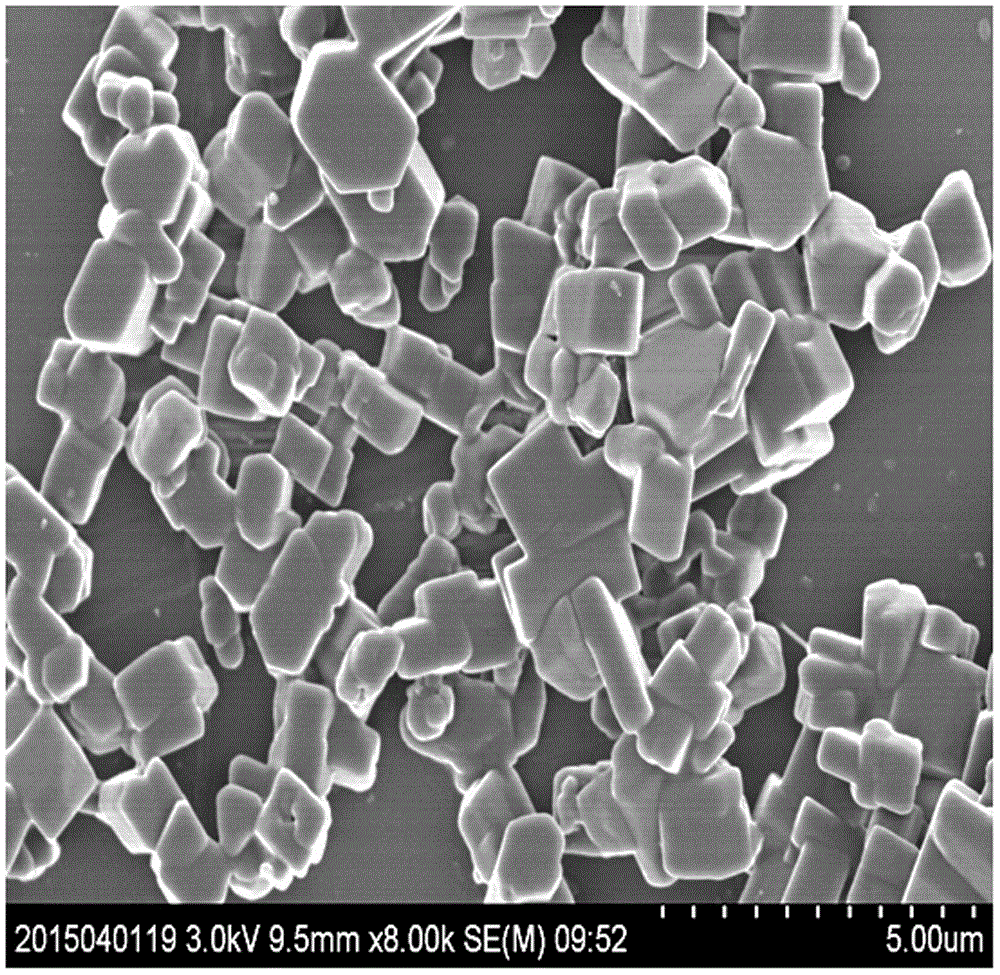
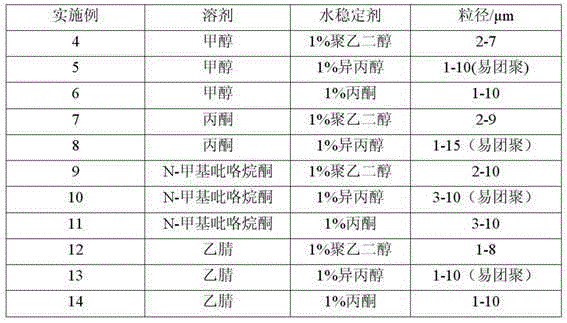
Method for preparing blonanserin micron drug through solvent method
A technology for blonanserin and drugs, applied in the field of chemical drug preparation, can solve the problems of easy agglomeration, uneven micron particles, irregular morphology, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 100ml of 1% polyethylene glycol water stabilizer to a 250ml Erlenmeyer flask, and place it in an ice-water bath at 0-4°C. Weigh 0.285 g (0.78 mmol) of blonanserin, dissolve it in 26 ml of ethanol, prepare a blonanserin ethanol solution with a concentration of 0.03 mol / l, and add it into a 50 ml constant pressure funnel. Place the constant pressure funnel on the Erlenmeyer flask filled with polyethylene glycol aqueous solution, turn on the ultrasonic cleaner, and slowly add the blonanserin ethanol solution dropwise (1ml / min) to the polyethylene glycol solution under ultrasonic conditions of 40KHZ frequency. In the ethylene glycol aqueous solution, the system became cloudy, and continued to sonicate for 30 minutes after the dropwise addition was completed. After the resulting mixed solution was left to stand for 1 h, it was subjected to high-speed centrifugation until it was completely separated, and the supernatant liquid was decanted. The obtained sample was washe...
Embodiment 2
[0025] Add 100ml of 1% polyethylene glycol water stabilizer to a 250ml Erlenmeyer flask, and place it in an ice-water bath at 0-4°C. Weigh 0.184 g (0.50 mmol) of blonanserin, dissolve it in 25 ml of ethanol, prepare a blonanserin ethanol solution with a concentration of 0.02 mol / l, and add it to a 50 ml constant pressure funnel. Place the constant pressure funnel on the Erlenmeyer flask filled with polyethylene glycol aqueous solution, turn on the ultrasonic cleaner, and slowly add the blonanserin ethanol solution dropwise (1ml / min) to the polyethylene glycol solution under the ultrasonic condition of 60KHZ frequency. In the ethylene glycol aqueous solution, the system became cloudy, and continued to sonicate for 30 minutes after the dropwise addition was completed. After the resulting mixed solution was left to stand for 2 h, it was centrifuged at 4000 r / min until it was completely separated, and the supernatant was poured off. The obtained sample was washed with distilled ...
Embodiment 3
[0027] Add 400ml of 1% polyethylene glycol water stabilizer to a 500ml Erlenmeyer flask, and place it in an ice-water bath at 15-20°C. Weigh 1.004 g (2.73 mmol) of blonanserin, dissolve it in 91 ml of ethanol, prepare a blonanserin ethanol solution with a concentration of 0.03 mol / l, and add it to a 100 ml constant pressure funnel. Place the constant pressure funnel on the Erlenmeyer flask filled with polyethylene glycol aqueous solution, turn on the ultrasonic cleaner, and slowly add the blonanserin acetone solution dropwise (1ml / min) to the polyethylene glycol solution under the ultrasonic condition of 40KHZ frequency. In the ethylene glycol aqueous solution, the system became cloudy, and continued to sonicate for 30 minutes after the dropwise addition was completed. After the resulting mixed solution was left to stand for 1 h, it was subjected to high-speed centrifugation until it was completely separated, and the supernatant liquid was decanted. The obtained sample was w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com