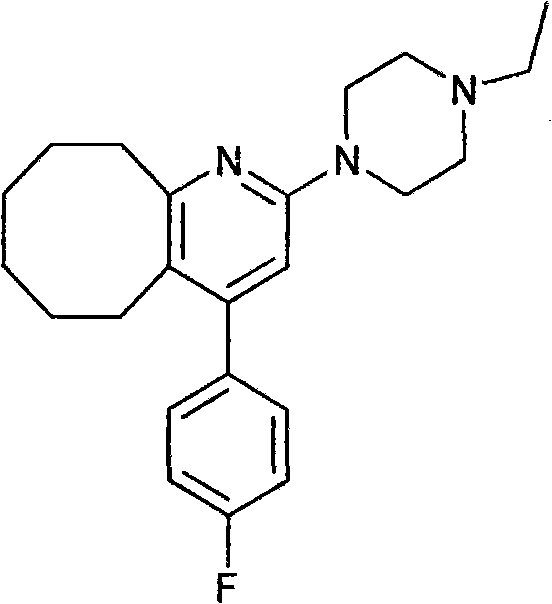
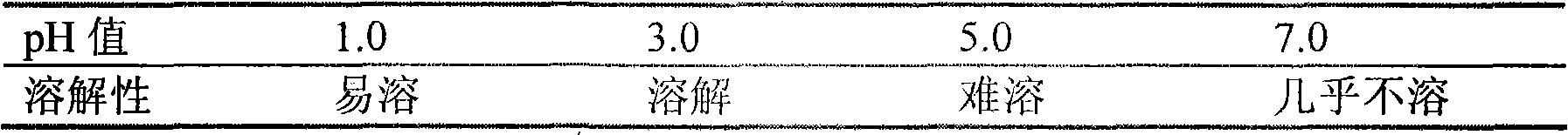Blonanserin-contained oral preparation for treating schizophrenia
A technology for schizophrenia and oral preparations, which is applied in the field of pharmaceutical preparations, can solve problems such as no production, and achieve the effects of good solubility, low dosage, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
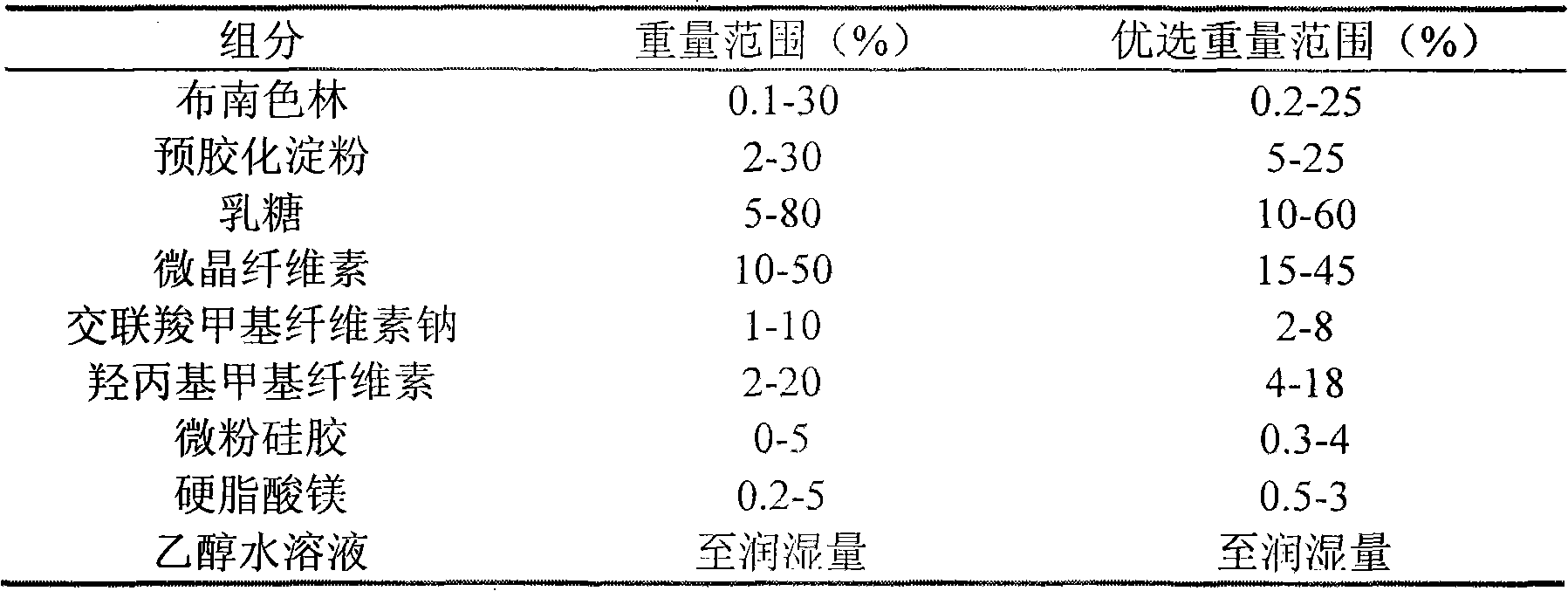
[0035] Composition and preparation method of compressed tablet with active ingredient 2 mg
[0036] Mix 2.0g of active ingredients, 20.0g of microcrystalline cellulose, 15.0g of pregelatinized starch, 36.0g of lactose, 6.0g of croscarmellose sodium, and 4.0g of hydroxypropylmethylcellulose, and mix them evenly, and use 50wt % ethanol aqueous solution is the soft material made of wetting agent, granulated with a 30-mesh screen, ventilated and dried at 55°C, granulated, added with 0.5g of micro-powdered silica gel and mixed evenly, pressed into about 1000 round tablets, each containing 2mg of active ingredient, namely Have to compress the tablet.
Embodiment 2
[0038] Composition and method of preparation of compressed tablet with active ingredient 4 mg
[0039] Mix 4.0g of active ingredients, 40.0g of microcrystalline cellulose, 20.0g of pregelatinized starch, 66.0g of lactose, 8.0g of croscarmellose sodium, and 8.0g of hydroxypropylmethylcellulose, and mix them evenly, and mix them with 50wt % ethanol water solution is the soft material made of wetting agent, granulated with a 30-mesh screen, ventilated and dried at 55°C, granulated, added with 1g of magnesium stearate and 1g of micro-powdered silica gel, mixed evenly, and pressed into about 1000 round tablets, each containing Active ingredient 4mg, that is, compressed tablets.
Embodiment 3
[0041] Composition and method of preparation of compressed tablet with 8 mg active ingredient
[0042] Mix 8.0 g of active ingredients, 60.0 g of microcrystalline cellulose, 30.0 g of pregelatinized starch, 100.0 g of lactose, and 12.0 g of croscarmellose sodium, and use 3 wt % hydroxypropyl methylcellulose aqueous solution as Soft material made of wetting agent, granulated with 30-mesh screen, ventilated and dried at 55°C, granulated, added with 2g of magnesium stearate and 1g of micro-powdered silica gel, mixed evenly, pressed into about 1000 round tablets, each containing 8mg of active ingredient, A compressed tablet is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com