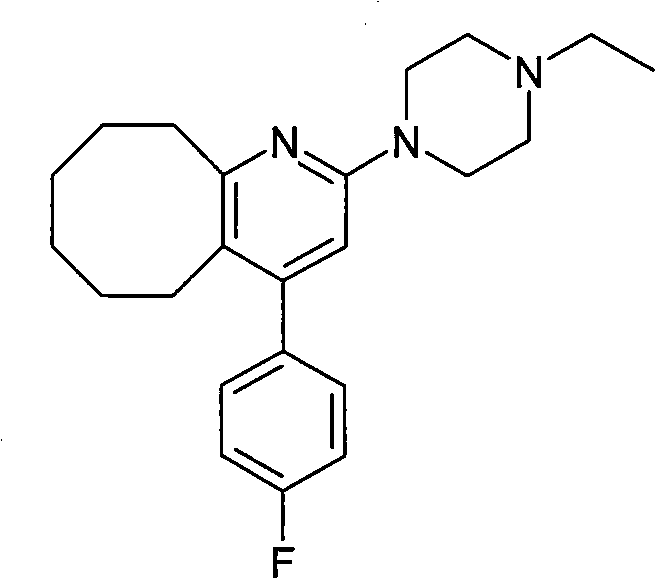
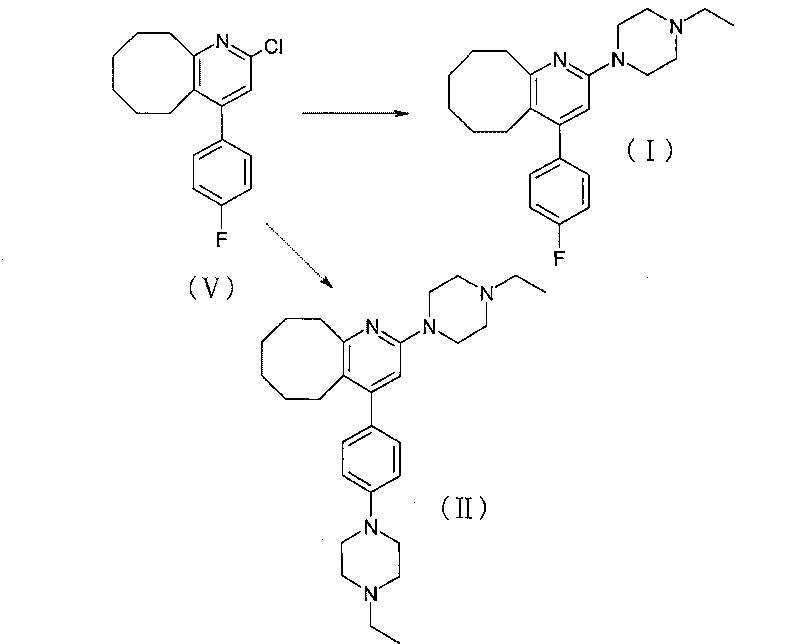
Method for preparing crystal form A of blonanserin
A crystal form, ethyl piperazine technology, applied in nervous system diseases, organic chemistry, drug combination and other directions, can solve problems such as high toxicity, and achieve the effect of safe production and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
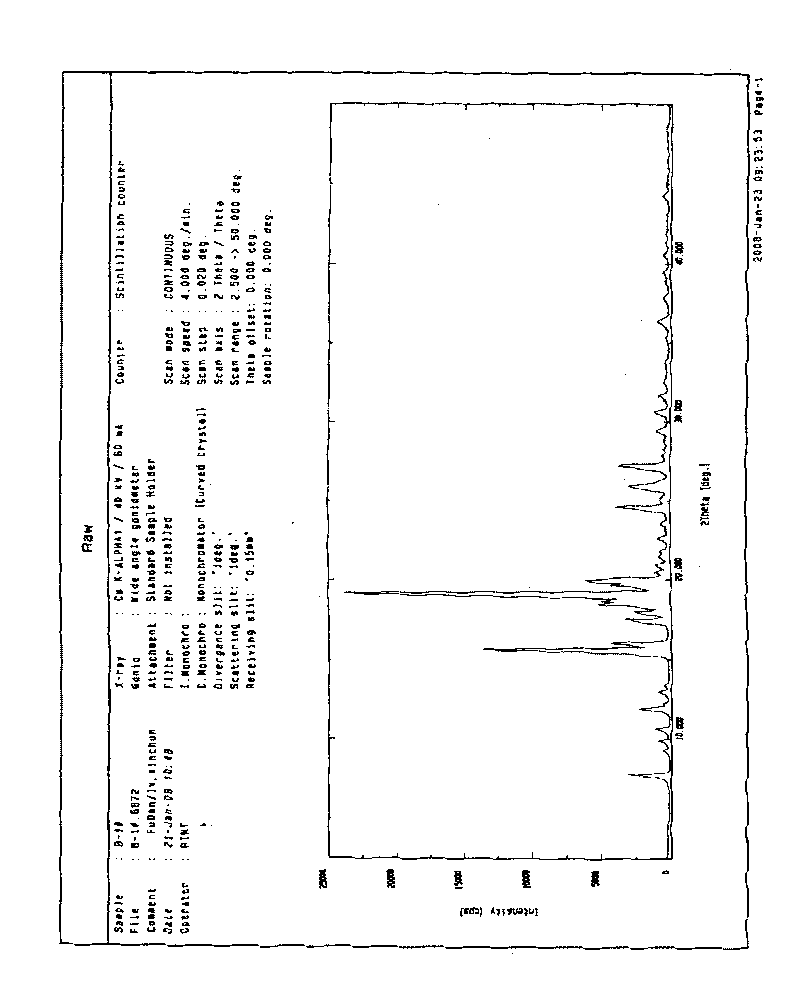
[0028] Add 1g of the crude branserin to 6ml of acetone, heat to reflux to dissolve, then cool to room temperature with stirring, and filter after 2 hours. Dry at 60°C. 0.8 g of a sample of Bronanserin crystal form A was obtained. The melting point is 126~127℃. The total related substances (determined by HPLC) were 0.17%, and the single impurity was 0.07%.
Embodiment 2
[0030] Add 1g of the crude branserin to 20ml of acetone, heat to reflux to dissolve, then cool to room temperature with stirring, and filter after 2 hours. Dry at 60°C. 0.78 g of a sample of bonanserin crystal form A was obtained. The melting point is 126~127℃. The total related substances (determined by HPLC) were 0.14%, and the single impurity was 0.06%.
Embodiment 3
[0032] Add 40g of the crude bulanserin to 350ml of acetone, heat to reflux to dissolve, then cool to room temperature with stirring, and filter after 2 hours. Dry at 60°C. 35 g of a sample of Bronanserin crystal form A was obtained. Melting point 125~126℃. The total related substances (determined by HPLC) were 0.30%, and the single impurity was 0.09%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com