Preparation method of blonanserin intermediate
A technology for blonanserin and intermediates, which is applied in the field of preparation of key intermediates of blonanserin, can solve the problems of incomplete reaction, low yield, long reaction time and the like, so as to reduce solvent loss and improve product yield. , The effect of product purity is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
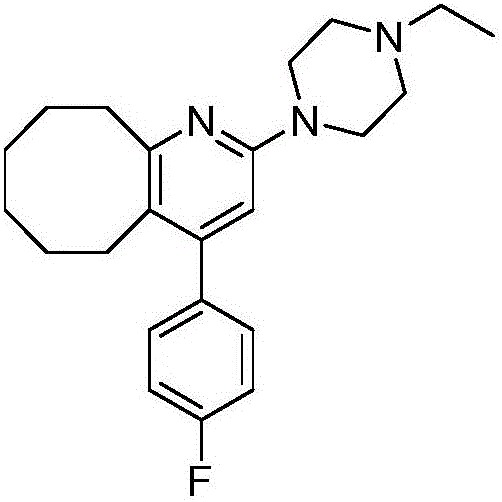
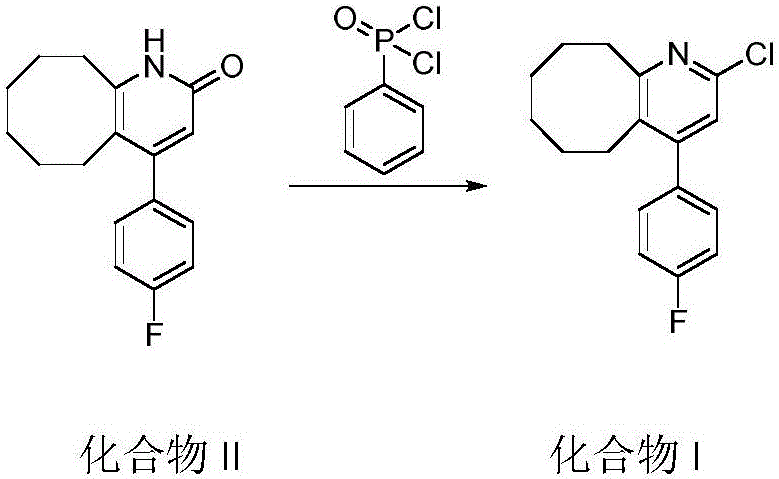
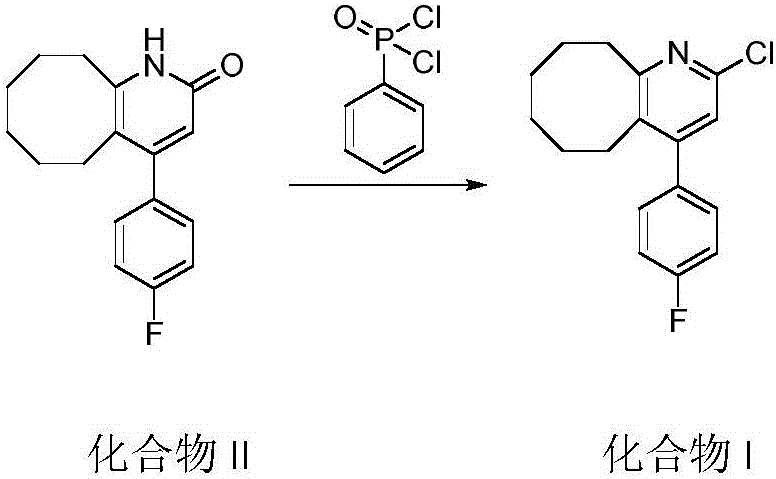
[0023] 4-(4-fluorophenyl)-3-5,6,7,8,9,10-hexahydrocyclooctanopyridin-2(1H)-one (120g) and phenylphosphonic dichloride (125mL ) into a 350mL thick-walled pressure-resistant bottle, heated at 120-130°C (external temperature) for 6 hours, and after the reaction was completed, phenylphosphonic dichloride was distilled under reduced pressure to obtain a black viscous liquid, which was poured into 2L of ice In the water mixture, add ammonia water to adjust the pH to 8.5, stir vigorously for 3-6 hours, an off-white solid precipitates, and filter to obtain a light gray or off-white solid (Compound I). Add compound I to ethanol (1.8 L), heat and stir to dissolve, add activated carbon (50 g), continue to stir at this temperature for 30 min, filter while hot, wash the filter cake with 400 mL of hot ethanol, the filtrate naturally crystallizes, and obtains white needles Crystals (110.8 g), yield 86.9%. HPLC purity 99.8%.
[0024] The proton nuclear magnetic resonance spectrum data of co...
Embodiment 2
[0027] 4-(4-fluorophenyl)-3-5,6,7,8,9,10-hexahydrocyclooctanopyridin-2(1H)-one (120g) and phenylphosphonic dichloride (187mL ) into a 350mL sealed tube, heated at 150°C (external temperature) for 4h, after the reaction was completed, phenylphosphonic dichloride was distilled under reduced pressure to obtain a black viscous liquid, which was poured into 2L of ice-water mixture, and ammonia water was added Adjust the pH to 9, stir vigorously for 3-6 hours, an off-white solid precipitates, filter or centrifuge to obtain a light gray or off-white solid. Then add ethanol (1.8L), heat and stir to dissolve, then add activated carbon (50g), continue stirring at this temperature for 30min, filter while it is hot, wash the filter cake with 400mL of hot ethanol, the filtrate crystallizes naturally to obtain white needle-shaped crystals (111.2 g), yield 87.2%. HPLC purity 99.8%.
[0028] The proton nuclear magnetic resonance spectrum data of compound I are as follows: δ6.99ppm (s, 1H); ...
Embodiment 3
[0031] 4-(4-fluorophenyl)-3-5,6,7,8,9,10-hexahydrocyclooctanopyridin-2(1H)-one (120g) and phenylphosphonic dichloride (155mL ) into a 350mL thick-walled pressure-resistant bottle, heated at 180-190°C (external temperature) for 4 hours, and after the reaction was completed, phenylphosphonic dichloride was distilled under reduced pressure to obtain a black viscous liquid, which was poured into 2L of ice water Add ammonia water to the mixture to adjust the pH to 8, stir vigorously for 3 to 6 hours, an off-white solid precipitates, filter or centrifuge to obtain a light gray or off-white solid. Then add ethanol (1.8L), heat and stir to dissolve, then add activated carbon (50g), continue stirring at this temperature for 30min, filter while it is hot, wash the filter cake with 400mL of hot ethanol, the filtrate crystallizes naturally to obtain white needle-shaped crystals (109.5 g), yield 85.5%. HPLC purity 99.7%.
[0032] The proton nuclear magnetic resonance spectrum data of com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com